HEPATOPROTECTIVE AND ANTIOXIDANT ACTIVITY OF SESBANIA GRANDIFLORA AGAINST CCl4-INDUCED HEPATIC INJURY IN RATS
HTML Full TextHEPATOPROTECTIVE AND ANTIOXIDANT ACTIVITY OF SESBANIA GRANDIFLORA AGAINST CCl4-INDUCED HEPATIC INJURY IN RATS
K. Padmalochana * 1 and M. S. Dhana Rajan 2
Sri Akilandeswari Women's College 1, Wandiwash - 604408, Tamil Nadu, India.
Jaya College of Arts and Science 2, Thiruninravur - 602024, Tamil Nadu, India.
ABSTRACT: A phytotherapeutic approach to modern drug development can provide many invaluable drugs from traditional medicinal plants. Medicinal plants have been considered as an important therapeutic aid for alleviating ailment of humankind. Numerous plants and polyherbal formulations are used for the treatment of liver diseases. This present investigation was aimed at assessing the hepatoprotective activity of aqueous, ethanol and acetone extract of Sesbania grandiflora leaves against carbon tetrachloride (CCl4) induced liver damage in Albino rats. Silymarin as a standard drug for comparing the activity. The activity was assessed by comparing the biochemical parameters in serum levels such as serum glutamate pyruvate transaminase, serum glutamate oxalate transaminase, total bilirubin, alkaline phosphatase of plant extracts treated group with carbon tetrachloride treated animals. Results showed, ethanolic extract treated group showed highly significant activity (P<0.001), whereas aqueous extract treated group has shown the significant (P<0.01) action but less compared with ethanolic extract, acetone treated group showed moderate action. Plant extracts restore biochemical enzymes and bring down to normal as compared to standard drug silymarin. This results shows and confirms the significant protective activity against CCl4 induced hepatotoxicity.
| Keywords: |
Phytotherapeutic, Sesbania grandiflora, Antioxidant, Carbon tetra chloride, Hepatotoxicity
INTRODUCTION: Liver is a very important organ in the human body. It regulates metabolic functions such as detoxification and plays a vital role in biochemical conversion. During the process of elimination, there is a chance of different accumulation kinds of toxic materials inside the hepatocytes, and there is a chance of liver infection, and hepatic disorders such as hepatitis 1. Liver diseases caused by various toxic chemicals, chemotherapeutic agents, excessive consumption of alcohol and microorganisms.
Hepatotoxicity is an acute adverse effect in the liver is caused by over dosages of drugs, toxic chemicals, viruses, bacteria and parasites 2. Hepatotoxicity is a slight change in hepatic structure and function which may result in hypertension, ascites, jaundice, increased bleeding and cause multiple metabolic changes affecting other organs 3, 4. The magnitude of derangement of the liver by disease or hepatotoxin is generally measured by the level of glutamate pyruvate transaminase (ALT), glutamate oxaloacetate transaminase (AST), alkaline phosphatase (ALP), bilirubin, albumin, and whole liver homogenate 4, 5.
CCl4 is a widely used industrial chemical and a potent hepatotoxin. It induces hepatotoxicity by producing free radical, putting oxidative stress hence causing lipid peroxidation in liver tissues, consequently necrotic liver damage 6, 7. Liver diseases such as hepatitis, cirrhosis and fatty liver are worldwide. Various commercial synthetic drugs are used to treat liver disorders also cause side effect on the liver. Hence, herbal drugs have become increasingly popular and their use is widespread. Herbal medicines have been used in the treatment of liver diseases for a long time. In India, numerous medicinal plants are used for the treatment of liver disorders 8. Hepatoprotective effect of some plants like Spirulina maxima 9 Eclipta alba 10, Boehmeria nivea 11, Cichorium intybus 12, and Picrorhiza kurroa 13, Boswellia serrata 4, Psidium quajava 14, Coccinia indica 15 has been well documented.
Sesbania grandifloraa Fig. 1 fast-growing tree belongs to the family, Fabaceae, is commonly known as agathi in regional language Tamil. The leaves, used as greens for cattle and poultry, have got anthelmintic property against selected helminths 16, 17. The bark, leaves, flowers, and roots are also used medically herbs distributed in the tropical regions of the globe 18. Juice of leaves and flowers is a popular remedy for nasal catarrh and headache when it is sniffed up the nostrils. Juice of the flowers is squeezed into the eyes to relieve the dimness of vision.
FIG. 1: SESBANIA GRANDIFLORA LEAVES
Juice of flower is ideal as expectorant 19. The leaves of the plant have been reported to have anxiolytic and anticonvulsant effect while the flowers have been reported to have anti-microbial activity 20. It shows hypolipemic, anti-ulcer and anti-inflammatory properties as well. Therefore, to justify the traditional claims, we have assessed the hepatoprotective effect of Sesbania grandiflora leaves extract in Albino rats using biochemical enzyme based analysis.
MATERIALS AND METHODS:
Chemicals: Analytical grade carbon tetrachloride, silymarin, and other chemicals were purchased from Himedia laboratories private limited, Mumbai. Sesbania grandiflora plant was collected from, Tiruvannamalai, South India.
Preparation of Plant Extracts: Sesbania grandiflora leaves were collected and shade dried at room temperature. The shade dried leaves were powdered and extracted by using aqueous, ethanol and acetone. Aqueous extracts were prepared by subjecting a 100 g of dried powdered leaves into 100 ml of distilled water and incubated in water bath shaker for 12 h at 40 °C. Ethanol and acetone extract prepared by the coarsely powdered leaves was extracted using Soxhlet and extracted with 80 % ethanol and 70 % acetone for 24 h at 60 °C and 55 °C, respectively. The extracted were collected and concentrated by drying under vacuum, and semisolid suspensions were obtained. These suspensions were used to assess hepatoprotective activity.
Experimental Design for Hepatoprotective Activity of Sesbania grandiflora: Adult male Wister albino rats maintained at the college weighing between 150-170g were used for the hepatoprotective studies. Animals were divided into six groups in six rats each:
Group I (Normal): Orally received distilled water for 7 days.
Group II (Induced): Orallyreceived carbon tetrachloride (2 g/kg body weight) only for 7 days.
Group III (Standard): Orally received Silymarin(20 mg/kg body weight) along with CCl4 (2g/kg body weight) for 7 days.
Group IV (Treatment): Orally received Aqueous leaf extracts (300 mg/kg body weight) along with CCl4 (2 g/kg body weight) for 7 days.
Group V (Treatment): Orally received ethanol leaf extracts (300 mg/kg body weight) along with CCl4 (2 g/kg/body weight) for 7 days.
Group VI (Treatment): Orally received acetone leaf extracts (300 mg/kg body weight) along with CCl4 (2 g/kg body weight) for 7 days.
Silymarin was used as a positive control for comparing the hepatoprotective potential of different leaves extract of Sesbania grandiflora.
Hepatoprotective Activity of S. grandiflora:
Collection of Blood and Biochemical Analysis: On the 8th day, all the animals were sacrificed, and blood samples were collected in glass tube from retro-orbital puncture to obtain hemolysis for 30 min at 37 °C. Serum glutamate oxaloacetate transaminase, serum glutamate pyruvate trans-aminase, serum bilirubin, and alkaline phosphatase and Serum protein 21, 22 were obtained from serum following centrifugation process was used for the biochemical analysis.
Antioxidant Activity of S. grandiflora:
Liver Homogenate Preparation: Liver homogenates were prepared by using a 100mM KCl buffer (pH7.0) containing 0.3mM EDTA and centrifuged at 6000 rpm for 45 min at 4 °C. After completion of the centrifugation process, collect the supernatant was used for estimation of antioxidant levels were analyzed superoxide dismutase (SOD) 23, catalase (CAT) 24 and glutathione peroxidase (GP) 25.
Statistical Analysis: The difference of biochemical parameters was measured using the statistical method, i.e. Analysis of Variance (ANOVA). Analysis of Variance refers to the examination of differences among the samples, and the results are expressed as mean± SEM and P<0.05, P<0.01, P< 0.001 was considered to be statistically significant.
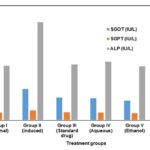
RESULTS AND DISCUSSION: The hepato-protective and antioxidant activity of S. grandiflora leaves extracts are shown in Fig. 2-4. The biochemical parameters such as serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transaminase, serum bilirubin, and alkaline phosphatase were estimated to assess the liver function. The marked increase in SGOT, SGPT, and ALP levels were observed in CCl4 treated group II animals are 94.98 ± 0.69, 29.98 ± 0.67 and 299.68 ± 0.12 IU/L, respectively. The increased level of SGOT, SGPT, ALP, and bilirubin is a conventional indicator of liver injury.
FIG. 2: HEPATOPROTECTIVE ACTIVITY IN CARBON TETRACHLORIDE INDUCED HEPATOTOXIC MODEL SHOWS CHANGES SERUM ENZYMES SGOT, SGPT, AND ALP IN SERUM
TABLE 1: HEPATOPROTECTIVE ACTIVITY IN CARBON TETRACHLORIDE INDUCED HEPATOTOXIC MODEL SHOWS CHANGES IN SERUM ENZYMES SGOT, SGPT, AND ALP IN SERUM
| Parameters | SGOT(IU/L) | SGPT(IU/L) | ALP(IU/L) |
| Group I (Normal) | 65.15±0.14*** | 23.15±0.17*** | 166.15±0.22*** |
| Group II (Induced) | 94.98±0.69* | 29.98±0.67* | 299.68±0.12* |
| Group III (Standard drug) | 69.42±0.38** | 24.42±0.33*** | 170.12±0.25*** |
| Group IV (Aqueous) | 67.48±0.39** | 23.48±0.38** | 178.48±0.28*** |
| Group V (Ethanol) | 59.59±0.76*** | 21.59±0.74*** | 164.51±0.17*** |
| Group VI (Acetone) | 62.58±0.34*** | 24.58±0.35** | 168.59±0.73*** |
*P <0.05, **P <0.01, ***P <0.001 value are considered statistically significant (BMRT)
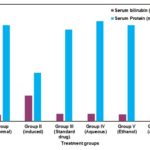
However, these levels were reversed to near normal levels of group I animals with the treatment of aqueous, ethanol and acetone extract of Sesbania grandiflora, which are statistically significant. The activities of extracts were comparable to a standard drug. These extracts have restored all the biochemical parameters levels in serum. And also the standard silymarin has restored the biochemical levels of SGOT, SGPT, and ALP significantly (P<0.01), i.e. 69.42 ± 0.38, 24.42 ± 0.33, and 170.12 ± 0.25 IU/L respectively in serum. In the case of bilirubin and total protein, there was a noticeable increase, i.e. in serum levels treating with CCl4. Treatment with aqueous, ethanol and acetone extract has reversed the serum bilirubin and total protein in serum levels to (0.50 ± 0.07 and 6.85 ± 0.12 mg/dl), (0.45±0.09 and 6.59 ± 0.32 mg/dl), (0.47 ± 1.06 and 6.26 ± 1.07 mg/dl), respectively which are statistically highly significant (P<0.001) when compared with CCl4 treated animals. The restoration of biochemical factors in serum was also noticed in treating with the standard drug silymarin (0.49 ± 0.04 and 6.26 ± 0.16 mg/dl). It is stipulated that the extract treated group was protected from hepatic cell damage caused by CCl4 induction.
FIG. 3: HEPATOPROTECTIVE ACTIVITY IN CARBON TETRACHLORIDE INDUCED HEPATOTOXIC MODEL SHOWS CHANGES SERUM BILIRUBIN AND TOTAL PROTEIN
TABLE 2: HEPATOPROTECTIVE ACTIVITY IN CARBON TETRACHLORIDE INDUCED HEPATOTOXIC MODEL SHOWS CHANGES SERUM BILIRUBIN AND TOTAL PROTEIN
| Parameters | Serum Bilirubin (mg/dl) | Serum Protein (mg/dl) |
| Group I (Normal) | 0.44±0.03*** | 6.58±0.06*** |
| Group II (Induced) | 1.76±0.10* | 3.32±0.04* |
| Group III (Standard drug) | 0.49±0.04*** | 6.26±0.16** |
| Group IV (Aqueous) | 0.50±0.07** | 6.85±0.12** |
| Group V (Ethanol) | 0.45±0.09*** | 6.59±0.32*** |
| Group VI (Acetone) | 0.47±1.06** | 6.26±1.07*** |
*P <0.05, **P <0.01, ***P <0.001 value are considered statistically significant (BMRT).
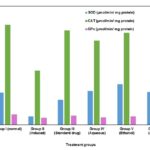
FIG. 4: ANTIOXIDANT LEVELS IN CARBON TETRA CHLORIDE INDUCED HEPATOTOXIC MODEL SHOWS CHANGES IN THE LEVELS OF SOD, CAT AND GPx
TABLE 3: ANTIOXIDANT LEVELS IN CARBON TETRA CHLORIDE INDUCED HEPATOTOXIC MODEL SHOWS CHANGES IN THE LEVELS OF SOD, CAT AND GPX
| Parameters | SOD (µmol/min/
mg protein) |
CAT (µmol/min/
mg protein) |
GPx (µmol/min/
mg protein) |
| Group I (Normal) | 18.91 ± 0.31*** | 58.99 ±4.80*** | 5.98 ± 2.46*** |
| Group II (Induced) | 04.94± 0.21* | 31.89±3.43* | 3.98± 0.35* |
| Group III (Standard drug) | 14.73 ± 0.39** | 55.67 ±3.13** | 5.34 ± 2.11** |
| Group IV (Aqueous) | 20.01 ± 0.17** | 49.68 ±0.55*** | 4.32 ± 1.92** |
| Group V (Ethanol) | 23.92 ± 0.27*** | 54.23 ±3.17*** | 4.84 ± 1.70*** |
| Group VI (Acetone) | 19.46 ± 0.11*** | 50.38 ±0.18*** | 4.42 ± 1.02*** |
*P<0.05, **P<0.01, ***P<0.001 value are considered statistically significant (BMRT).
The extract at a dose of 300 mg/kg body wt. exhibited orally, the significant protective effect by lowering the serum levels of transaminases (SGOT and SGPT), bilirubin and alkaline phosphatase (ALP). The effects produced were comparable to that of standard hepatoprotective agent silymarin. In ethanol extract treated animals; the toxicity effect of carbon tetrachloride was controlled significantly by restoration of the levels of serum bilirubin and enzymes as compared to the normal and standard drug silymarin-treated groups. Antioxidant activities of hepatic SOD, CAT and GPx were estimated and shown in Table 3, Fig. 4 SOD, CAT, and GPx activities were significantly (p<0.001) enhanced only in the orally received ethanol extract of S. grandiflora leaves. The antioxidant activities of aqueous, ethanol and acetone extract show significant activity near to the normal group of animals.
CONCLUSION: In the present report stated that the aqueous, ethanol and acetone extract of commonly available plant Sesbania grandiflora leaves was extensively investigated for its hepatoprotective potential against CCl4 induced hepatotoxicity. There was a significant increase in serum levels of bilirubin, alanine transaminase, aspartate transaminase and alkaline phosphatase with a decrease in total protein level, in the CCl4 treated animals, reflecting liver injury.
In the extracts treated animals, there was a decrease in serum levels of the markers and a significant increase in total protein, indicating the recovery of hepatic cells. A strong conclusion can be drawn that, extract of Sesbania grandiflora possess the most significant (P<0.001) hepatoprotective activity compared with the standard drug silymarin so that the development of medicines using the extracts of plant materials or bioactive compounds with standards of safety and efficacy can revitalize the treatment of liver disorders and hepato-protective activity.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Aneja S, Vats M, Aggarwal S and Sardana S: Phyto-chemistry and hepatoprotective activity of aqueous extract of Amaranthus tricolor roots. Journal of Ayurveda & Integrative Medicine 2013; 4(4): 211-215.
- Bhawna S and Kumar SU: Hepatoprotective activity of some indigenous plants, International Journal of Pharm Tech Research 2009; 1(4): 1330-1334.
- Fernandez-Checa JC, Hirano T, Tsukamoto H and Kaplowitz N: Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol 1993; 10: 469-475.
- Ibrahim M, Uddin KZ and Narasu ML: Hepatoprotective activity of Boswellia serrate extracts: in-vitro and in-vivo International J of Pharmaceutical Applications 2011; 2(1): 89-98
- Schuppan D, Athionson J, Ruehl M and Riecken EO: Alcohol and liver fibrosis-pathobiochemistry and treatment. Z Gastroenterol 1995; 33: 546-550.
- Ma KM, Nyunt N and Tin M: The protective effects of Eclipta alba on carbon tetrachloride induced acute liver damage. Toxicol and Applied Pharmacol 1978; 45: 723-728.
- Ram VJ and Goel A: Curr Med Chem 1999; 6: 217-254.
- Sharma SK, Ali M and Gupta J: Evaluation of Indian herbal hepatoprotective drugs. Recent progress in medicinal plants (Phytochemistry and Pharmacology). Houston: Research Periodicals and Book Publishing House; Vol. 2, 2002: 253-70.
- Torres-Duran PV, Miranda-Zamora R, Paredes-Carbajal MC, Mascher D, Ble-Castello J, Diaz- Zagoya JC and Juarez-Oropeza MA: Studies on the preventive effect of Spirulina maxima on fatty liver development induced by carbon tetrachloride, in the rat. J Ethnopharmacol 1999; 64: 141-147.
- Saxena AK, Singh B and Anand KKL Hepatoprotective effects of Eclipta alba on Subcellular levels in rats. J Ethnopharmacol 1993; 40: 155-161.
- Lin CC, Yen MH, Lo TS and Lin JM: Evaluation of the hepatoprotective and Antioxidant activity of Boehmeria nivea nivea and B. Nivea var. tenacisssima. J Ethnopharmacol 1998; 60: 9-17.
- Zafar R and Mujahid Ali S: Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus J Ethnopharmacol 1998; 63:227-231.
- Saraswat B, Visen PK, Patnaik GK and Dhawan BN: Ex- vivo and in-vivo Investigations of picroliv from Picrorhiza kurroa in alcohol intoxication model in rats. Journal Ethnopharmacol 1999; 66: 263-269
- Roy CK, Kamath JV and Asad M: Heaptoprotective acitivity of Psidium quajava leaf extract. Indian Journal of Experimental Biology 2006, 44: 305-311.
- Shyam Kumar B, Gnanasekaran D, Jaishree V and Channabasavaraj KP: Hepatoprotective activity of Coccinia indica leaves extract. Int J Pharm Biomed Res 2010; 1(4): 154-156
- Sharma PC, Yelre HB and Dennis JJ: Database on medicinal plants used in CCRAS publication. Ayurveda, 2005; 1-6.
- Vaidhyaratnum PSV: Indian medicinal plants. A compendium of 500 species. Orient Longman, Madras, India, Vol. V, 1996: 17 -118.
- Berhaut J: Flore illustrated of Senegal. Tome V Government of Senegal, 1976: 505-520
- Duke JA: Handbook of Energy Crops. Purdue University 1983.
- Krasaekoopt W and Kongkarchanatip A: Antimicrobial properties of Thai traditional flower vegetable extracts. AUJT 2005; 9(2): 71-74.
- Patel BA, Patel JD and Raval BP: Hepatoprotective activity of Sachharum officinarum against paracetamol-induced hepatotoxicity in rats. Int J Pharm Sci Res 2010; 1: 102-
- Manokaran S, Jaswanth A, Sengottuvelu S, Nandhakumar J, Duraisamy R and Karthikeyan D: Hepatoprotective activity of Aerva lanata against paracetamol-induced hepatotoxicity in rats. Res J Pharm Tech 2008; 1: 398-400.
- Ukeda H, Maeda S, Ishii T and Sawamura M: Anal Biochem 1997; 251: 206-209.
- Aebi H: Catalase in-vitro. Methods in Enzymol 1984; 105: 121-126.
- Okawa M, Kinjo J, Nohara T and Ono M: DPPH (1,1-diphenyl-2- picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol Pharm Bull 2001; 24: 1202-5.
How to cite this article:
Padmalochana K and Rajan MSD: Hepatoprotective and antioxidant activity of Sesbania grandiflora against CCl4-induced hepatic injury. Int J Pharmacognosy 2015; 2(2): 71-76. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.2(2).71-76.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
71-76
609
2696
English
IJP
K. Padmalochana* and M. S. D. Rajan
Department of Biochemistry, Sri Akilandeswari Women's College, Wandiwash, Tamil Nadu, India.
kpadmalochana@gmail.com
31 December 2014
19 January 2015
17 January 2015
http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.2(2).71-76
01 February 2015