HEPATOPROTECTIVE ACTIVITY OF MORUS ALBA (LINN). LEAVES EXTRACT AGAINST PARACETAMOL INDUCED HEPATOTOXICITY IN RATS
HTML Full TextHEPATOPROTECTIVE ACTIVITY OF MORUS ALBA (LINN). LEAVES EXTRACT AGAINST PARACETAMOL INDUCED HEPATOTOXICITY IN RATS
M. G. Hogade * 1 and S. S. Kuthar 2
Department of Pharmacognosy and Phytochemistry 1, Vilasrao Deshmukh Foundation, Group of Institutions, VDF School of Pharmacy, New MIDC, Airport Road, Latur - 413531, Maharashtra, India.
Department of Pharmaceutical Chemistry 2, MBES College of Pharmacy, Barshi Road, Near Woman’s Polytechnic, Latur - 413531, Maharashtra, India.
ABSTRACT: Aim: To investigate the hepatoprotective activity of Morus alba Linn. alcoholic leaves extracts against paracetamol-induced hepatitis in rats. Materials and Methods: The Morus alba leaves extracted with alcoholic (ALE), and water extract (AQE) against Paracetamol induced hepatotoxicity and using Standard drug is Liv-52. Preliminary phytochemical tests were done. Results: The ALE showed presence of alkaloids, flavonoids, carbohydrates, tannins, and steroids, did not produce any mortality. Paracetamol produced significant changes in biochemical parameters (increases in serum glutamate pyruvate transaminase (SGPT), Serum glutamate oxaloacetate transaminase (SGOT), alanine phosphatase (ALP) and serum bilirubin.), histological (damage to hepatocytes) using Standard drug Liv-52. Pre-treatment with ALE extract significantly prevented the biochemical and histological changes induced by Paracetamol in the liver. Conclusion: The present study shows that the ALE extract possessed hepatoprotective activity.
| Keywords: |
Hepatoprotective, Paracetamol, Morus alba Linn.
INTRODUCTION: The liver is the key organ regulating homeostasis in the body. It is involved with almost all the biochemical pathways related to growth, fight against diseases, nutrient supply, energy provision, and reproduction 1. The liver is expected not only to perform physiological functions but also to protect against the hazards of harmful drugs and chemicals. In spite of tremendous scientific advancement in the field of hematology in recent years, liver problems are on the rise.
Jaundice and hepatitis are two major hepatic disorders that account for a high death rate 2. Presently only a few hepatoprotective drugs and that too from natural sources are available for the treatment of liver disorders 3. Morus alba Linn (Moraceae) is also known as Tut in India. The white mulberry has a long history of medicinal use in Chinese medicine; almost all parts of the plant are used in one way or another.
Recent research has shown improvements in elephantiasis when treated with leaf extract injections and in tetanus oral doses of the sap mixed with sugar 4. The fruit has a tonic effect on kidneys 5. It is used in the treatment of urinary incontinence, dizziness, tinnitus, insomnia due to anemia, neurasthenia, hypertension, diabetes, premature graying of the hair, and constipation in the elderly 6.
The leaves are showing analgesic and anti-inflammatory activity of hydroalcoholic extract of leaves 7. Phytochemical review shows the presence of tannins, Vitamin A, flavonoid, thiamine, protein, carbohydrates 8. It has been used in the indigenous system of medicine for cooling, acrid, purgative, diuretic, laxative, anthelmintic, brain tonic, anti-bacterial, hepatopathy properties. They are useful in vitiated conditions of vata and pitta, burning sensation 9.
Hence the present study was aimed to investigate the hepatoprotective activity of alcoholic leaves extracts of Morus alba L. against paracetamol-induced hepatitis in rats
MATERIALS AND METHODS:
Plant Collection and Authentication: The leaves of Morus alba Linn. were collected from Ramling mudgad Dist.-Latur (Maharashtra); were authenticated by Dr. Harsha Hegade. Research officer Indian Council of Medical Research, Belgaum. A voucher specimen has been deposited at the herbarium of RMRC-465.
Preparation of Extracts: The plant material (leaves) were dried for several days and powdered with the help of an electric grinder. The course material was extracted in a Soxhlet extractor with alcohol (90%) and aqueous. The extracts were dried at 50 ºC in a water bath. The percentage yields of the alcoholic extract are 8.231%, and the aqueous extract is 11.10%.
Chemicals: Liv-52 obtained from Himalaya drug, Bangalore. All other chemicals used were of analytical grade.
Experimental Animals: Swiss albino mice (18-20 g) and Wistar rats (150–200 g) of either sex were procured from Sri Venkateshwara Enterprises, Bangalore, and were acclimatized for 10 days under standard housing condition maintained at a room temperature of 24 ± 1 ºC; related humidity 45-55% with 12:12 h light/dark cycle. The animals were habituated to laboratory conditions for 48 hrs prior to the experimental protocol to minimize any nonspecific stress.
LD50 Determination: Acute oral toxicity (AOT) of ALE and AQE were determined using nulliparous, non-pregnant female mice.
The animals were fasted for 3 h prior to the experiment and were administered with a single dose of extracts dissolved in 2% w/v Tween 80 and observed for mortality for upto 48 hours.
Based on the short-term toxicity, the dose of next animal was determined as per OECD guidelines 10. All the animals were also observed for long-term toxicity. The LD50 of the test extracts was calculated using ‘AOT 425’ software provided by Environmental Protection Agency, USA.
Hepatoprotective Activity: The method of S. Ramachandra S et al., was used in the study. Animals were divided into seven groups of 6 animals each. The first group received saline 1 ml/kg for one week (control).
Group II received saline 1 ml/kg for one week (positive control). The group's III, IV, V, VI, and VII received Liv-52 (4 ml/kg p.o.) and 150 mg/kg, 300 mg/kg and 175 mg/kg, 350 mg/kg of Morus alba ethanolic extract respectively once a day for seven days.
On the fifth day, after the administration of the respective treatments, all the animals of groups II, III, IV, V, VI and VII were administered with paracetamol 2 g/kg orally. On the seventh day after 2 h of respective treatments the blood samples were collected for the estimation of biochemical marker enzymes. Then animals under ether anesthesia were sacrificed. The livers from all the animals were collected, washed and used for the estimation 11.
Blood Biochemistry: Blood samples were collected in glass tube from retro orbital puncture to obtain haemolysis-free clear serum for the analysis of SGOT and SGPT12. ALP13. and Bilirubin 14 by standard method.
Histopathology: Histopathology of the liver was carried out by a modified of Luna 15. In brief, the autopsied livers were washed in normal saline and fixed in 10% formalin for 2 days, followed with bovine solution for 6 h. Then the livers were paraffin-embedded, and 5 µ thickness microtone sections made 16. The section was processed in alcohol-xylene series and stained with haemato-xylin and eosin. The slides were studied under a light microscope for any histological damage/ protection.
Statistical Analysis: The data obtained were analyzed by One Way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using a computerized program. P-value <0.05 or was taken as the criterion of significance.
RESULTS: Preliminary phytochemical studies revealed the presence of alkaloids, carbohydrates, flavonoids, tannins, steroids in ALE. While alkaloids, carbohydrates, flavonoids, and tannins were noticed in AQE.
The ALE was found to be nontoxic upto a dose of 3000 mg/kg, and LD50 of AQE was found to be 3500 mg/kg. Treatment of rats with Paracetamol produced an increase in the weight and volume of wet liver. Rats were pretreated with Liv-52, ALE and AQE. ALE and AQE showed a significant effect. Paracetamol administration resulted in significant elevation of SGOT, SGPT, ALP and serum Bilirubin. Biochemical parameters were found to be decreased compared to the normal control group due to pre-treatment with Liv-52, ALE, and AQE, which significantly prevented the biochemical changes induced by Paracetamol. The hepatoprotective effect offered by ALE was found to be significantly greater than AQE treatment Table 1.
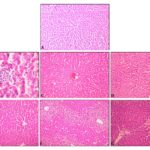
Hepatocytes of the normal control group showed normal lobular architecture of the liver. In the Paracetamol treated group, the liver showed microvascular fatty changes, and the hepatocytes were surrounded by large No. of fat droplets; Liv-52, ALE, and AQE pretreated groups showed minimal fatty changes Fig. 1 and their lobular architecture was normal, indicating the hepato-protective effect of these extracts. However, ALE showed more microvascular fatty changes Fig.1 than AQE. The hepatoprotective activity of the extracts was in the order of Liv 52 > ALE > AQE.
FIG 1: HISTOLOGY OF LIVER SHOWING NORMAL HEPATOCYTE (A), PARACETAMOL INDUCED MICROVASCULAR FATTY CHANGES SURROUNDING BY A LARGE NUMBER OF SMALL FATTY DROPLETS (B), HEPATOCYTES IN GROUPS TREATED WITH LIV-52 (C), ALE (150 & 300 MG/KG) (D &E) AND AQE (175 & 350 MG/KG) (F & G) PRIOR TO ADMINISTRATION OF PARACETAMOL SHOWING MINIMAL FATTY CHANGES.
FIG. 2: EFFECT OF PEE, CHE, ALE AND AQE ON SERUM BIOCHEMICAL PARAMETERS AGAINST PARACETAMOL (2 G/KG, S.C) INDUCED LIVER DAMAGE. (A) REPRESENTATION OF ALANINE AMINOTRASFERASE. (B) REPRESENTATION OF ASPARTATE AMINOTRASFERASE. (C) REPRESENTATION OF ALKALINE PHOSPHATASE. (D) REPRESENTATION OFSERUM BILIRUBIN.
TABLE 1: EFFECT OF MORUS ALBA L. LEAVES EXTRACTS ON PARACETAMOL- INDUCED HEPATOTOXICITY IN RATS
| Treatment | Dose (ml/kg) | SGOT (U/L) | SGPT (U/L) | ALP (U/L) | Serum bilirub in (mg/dl) |
| Control | 1 | 31.42 ± 0.7574 | 88.50 ± 2.766 | 91.17 ± 5.498 | 0.2733 ± 0.1258 |
| Liv-52 | 4 | 47.17 ± 1.851 | 100.2 ± 3.260 | 107.5 ± 3.631 | 0.2367 ± 0.015 |
| Paracetamol | 2 g/kg s.c | 400.25 ± 1.302 | 282.3 ±2.290 | 431.3 ±1.116 | 0.7150± 0.0217 |
| ALE | 150 | 114.7 ±1.926* | 287.7 ±3.809* | 213.3 ± 4.410* | 1.882 ± 0.0452* |
| 300 | 52.33±1.909** | 255.2 ±4.175** | 184.5 ±1.727** | 1.015 ± 0.0506** | |
| AQE
|
175 | 195.0 ±1.880 | 361.8 ±3.208 | 385.5 ±8.884 | 2.175 ±0.0495 |
| 350 | 108.0 ± 3.152* | 309.5 ± 3.160* | 307.5 ±3.263* | 1.740 ±0.1145* |
Results are expressed as mean ± SEM, n= 6, (P**< 0.01) vs. Paracetamol treated group using one–way ANOVA followed by Tukey Kramer’s post hoc test.
DISCUSSION: The liver can be injured by many chemicals and drugs. In the present study, Paracetamol was selected as a hepatotoxicant to induce liver damage since it is clinically relevant. Paracetamol produces a constellation of dose-related deleterious effects in the liver 17.
During hepatic damage, cellular enzymes like SGOT, SGPT, ALP and serum Bilirubin present in the liver cell, leak into the serum, resulting in an increase concentration 18. Histological changes such as steatosis (fatty changes in hepatocytes) and perivenular fibrosis were observed in paracetamol control group. Both the extracts prevented these histological changes, further indicating their hepatoprotective activity. All the histological changes observed were in correlation with the biochemical and functional parameters of the liver.
It can be concluded that Morus alba leaves extracts viz. ALE and AQE possess a protective effect against Paracetamol-induced hepatotoxicity in rats, but ALE shows a more significant effect as evidenced by the biochemical, functional, and histological parameters.
ACKNOWLEDGEMENT: We are thankful to the President and Principal, VDF School of Pharmacy, Latur, for providing the facilities to carry out the research work.
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Ward FM and Daly MJ: Hepatic disease. In: Walker R, Edwards C, editors. Clinical Pharmacy and Therapeutics. Churchill Livingston: New York 1999; 195-12.
- Pang S, xin X and Stpierre MV: Determinants of Metabolic Disposition. Rev Pharma Tox 1992; 32: 625-26.
- Ross MH, romrell LJ and Kaye GI: Histology a text and atlas. Wilian and Wilkin: Baltimore1996; 245.
- Bown D: Encyclopaedia of Herbs and their Uses, Dorling Kindersley, London 1995; 20-31
- Duke JA and Ayensu ES: Medicinal Plants of China, Reference Publications, Inc, China 1985; 20-4
- Yeung Him-Che: Handbook of Chinese Herbs and Formulas. Institute of Chinese Medicine, Los Angeles 1985; 320-24.
- Vaghasiya RG, Banerjee A and Shrivastava N: Indian Drugs 2007; 44(5): 364-67.
- Wealth of India Raw Materials, CSIR, New Delhi 1962; 435-36.
- Arya Vaidya Sala: Indian Medicinal Plant. Orient Longman Limited, Chennai 1997; 14: 65-67.
- OECD 2001-gudeline on acute oral toxicity (AOT). Environmental heaith and safety monograph series on testing and adjustment. No. 425.
- Setty SR, Quereshi AA and Swamy AHMV: Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia 2007; 78: 451-54
- Reitman S and Frankel S: A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. American Journal clinical Pathology 1957; 28: 56-63.
- Walter K and Schutt C: Acid and alkaline phoshatases in serum. In: Verlag Chemic Weinheim, In: Hans Ulrich Bergmeyer (Ed.), Method of Enzymatic Analysis. Vol. 2. Academic Press Inc., New York 1974; 856-64.
- Malloy HT and Evelyn KA: The determination of bilirubin with the photoelectric colorimeter. Journal of Biological Chemistry 1937; 119: 481-90.
- Luna LG: Manual in histology and staining method. McGraw Hill: New York 1999; 96.
- Krajian AA: Tissue cutting and staining. In: Frankel, S., Reitman, S. (Eds.), Gradwohl’s Clinical Laboratory Method and Diagnosis. The CV. Mosby Co., Saint Louis, USA 1963; 1639.
- Diadelis R, Jan NM and Commandeur ED: Environ Toxicol Pharmacol Sect., Eur J Pharmacol 1995; 293-01.
- Deb AC: Fundamental of Biochemistry. 7th New central book Agency: Kolkata 1998.
How to cite this article:
Hogade MG and Kuthar SS: Hepatoprotective activity of Morus alba (linn). leaves extract against paracetamol induced hepatotoxicity in rats. Int J Pharmacognosy 2021; 8(3): 124-28. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.8(3).124-28.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
5
124-128
972
817
English
IJP
M. G. Hogade * and S. S. Kuthar
Department of Pharmacognosy and Phytochemistry, Vilasrao Deshmukh Foundation,Group of Institution, VDF School of Pharmacy, New MIDC, Latur, Maharashtra, India.
maheshhogade@gmail.com
19 February 2021
28 March 2021
30 March 2021
10.13040/IJPSR.0975-8232.IJP.8(3).124-28
31 March 2021