HEPATOPROTECTIVE ACTIVITY OF ETHANOLIC EXTRACT FROM THE STEM BARK OF SYNEDRELLA NODIFLORA ON CARBON TETRACHLORIDE INDUCED HEPATOTOXICITY IN SWISS ALBINO RATS
HTML Full TextHEPATOPROTECTIVE ACTIVITY OF ETHANOLIC EXTRACT FROM THE STEM BARK OF SYNEDRELLA NODIFLORA ON CARBON TETRACHLORIDE INDUCED HEPATOTOXICITY IN SWISS ALBINO RATS
Tasmiatul Kabir * 1 and Md. Rokibul Hossain 2
Department of Pharmacy 1, University of Asia Pacific, Dhanmondi, Dhaka, Bangladesh.
Department of Pharmacy 2, Southeast University, Banani, Dhaka, Bangladesh.
ABSTRACT: The ethanol extracts of stem bark of Synedrella nodiflora belonging to the family of Asteraceae was studied for hepatoprotective activity against Swiss Albino Rats with liver damage induced by carbon tetrachloride (CCl4). It was found that the ethanol extract of Synedrella nodiflora at a dose of 250 mg/kg body weight exhibited significant protective effect by lowering the serum level of alanine aminotransferase (ALT) or serum Glutamate Pyruvate Transferase (SGPT), and 100 mg/kg body weight exhibited significant protective effect by lowering both the serum levels of alanine aminotransferase (ALT) or serum Glutamate Pyruvate Transferase (SGPT), aspartate aminotransferase (AST) or serum glutamate oxaloacetate transaminase (SGOT). The highest hepato-protective activity was observed for ethanol extract of Synedrella nodiflora at a dose of 250 mg/kg body weight, and the reduction of serum level of ALT, AST and serum bilirubin were 60.918 ± 0.7936, 81.479 ± 2.85039 and 0.5205 ± 0.0249 respectively. Since, result of biochemical studies of blood sample of carbon tetrachloride treated rats showed significant increase in the level of serum enzyme activities , reflecting the liver injury caused by CCl4 and blood sample from the animals treated with the ethanol extract of Synedrella nodiflora showed significant decrease in the level of serum marker, indicating the protection of hepatic cells, the extract of above plant could afford significant dose-dependent protection against CCl4 induced hepatocellular injury.
| Keywords: |
Synedrella nodiflora, Carbon tetrachloride, Hepatotoxicity, Serum Bilurubin, Alaninenamino transferase, Aspartate aminotransferase
INTRODUCTION: The liver is the second largest organ in the body, and is often seen as the most important one. The liver receives a dual blood supply with about 20% of blood coming from the hepatic artery and 80% from the portal circulation.
The blood flow to the liver is around 20 to 25% of the total cardiac output. Toxins, infectious agents, medications, and serum inflammatory mediators may result in a diverse range of disease processes, leading to loss of normal histological architecture, reduced cell mass, and loss of blood flow.
Consequently, functional liver capacity may be lost. Efforts have been made to search for effective hepatoprotective agents. However, no effective hepatoprotective therapies are available until now. Therefore, the prevention of liver diseases has a great significance both in theory and in practice 1.
Herbal medicines are in great demand in the developed as well as developing countries for primary healthcare because of their wide biological and medicinal activities, higher safety margins and lesser costs 2. Modern drugs have very little to offer for the alleviation of hepatic ailments, whereas most important representatives of phytoconstituents used for liver diseases chiefly on regional basis include drugs like silymarin (Silybum marianum) and catechin (Anacardium occidentalis) in Europe, Glycyrrhizin (Glycyrrhiza glarbra) in Japan and chizandrins (Schizandra chinesis) in China 3. Herbal drugs have gained importance and popularity in recent years because of their safety, efficacy, and cost-effectiveness. The association of medical plants with other plants in their habitat also influences their medicinal values in some cases. One of the important and well-documented uses of plant products is their use as hepatoprotective agents. Hence, there is an ever-increasing need for safe hepatoprotective agent 4.
Carbon tetrachloride (CCl4) is used as a model to study hepatotoxic effects and causes liver damage through a number of mechanisms. Liver cell injury induced by carbon tetrachloride involves the metabolism of carbon tetrachloride initially to trichloromethyl free-radical by the mixed function oxidase system of the endoplasmic reticulum. It is postulated that secondary mechanism slinks carbon tetrachloride metabolism to the widespread disturbances in hepatocyte function. These secondary mechanisms could involve the generation of toxic products arising directly from carbon tetrachloride metabolism or peroxidative degeneration of membrane lipids. The possible involvement of radicals species such as trichloromethyl (CCl3), trichloromethyl peroxy (OOCCl3), and chlorine (Cl) free radicals, as well as phosgene and aldehydic products of lipid peroxidation, as toxic intermediates are discussed. Data do not support the view that an increase in cytosolic free calcium is important in the toxic action of carbon tetrachloride or bromotrichloro-methane. Also, carbon tetrachloride-induced inhibition of very low density lipoprotein secretion by hepatocytes is not a result of elevated levels of cytosolic free calcium 5.
The plant Synedrella nodiflora (locally known as Dudia) belongs to the family Asteraceae. Extract of leaves and steam exhibit potential antimicrobial activity 6. Ethanol extracts of the show the antioxidant activity 7. Thus, the objective of the present study was designed to test the hepatoprotective activity of the extract of plant material of above-specified plant against carbon tetrachloride-induced liver damaged in rats.
Medicinal Value of Examined Plant:
- Synedrella nodiflora pendula leaves have antidiarrheal, analgesic activities of methanolic extract 8.
- Various parts of Synedrella nodiflora pendula has Antimicrobial activity 9.
- It has anti-hyperglycemic effect against sucrose loading induced hyperglycemia 10.
The liver is a vital organ present in vertebrates and some other animal. It has a wide range of function, including detoxification, protein synthesis and production of biochemical’s necessary for digestion. The liver is necessary for survival; there is currently no way to compensate for the absence of liver function long term, although liver dialysis can be used short term.
This organ plays a major role in metabolism and has some functions in the body, including glycogen storage, decomposition of red blood cells, plasma protein synthesis, hormone production, and detoxification. It produces bile, an alkaline compound which aids in digestion via the emulsification of lipids. The liver’s highly specialized tissues regulate a wide variety of high-volume biochemical reaction, including the synthesis and breakdown of small and complex molecules, many of which are necessary for vital function 11.
MATERIALS AND METHODS:
Materials:
Collection and Identification of the Plant Sample: Fresh and mature steam were collected from a reliable source and identified by a taxonomist. An accession number was given by herbarium for confirming actual classification Table 1.
TABLE 1: COLLECTION AND IDENTIFICATION OF THE PLANT SAMPLE
| Scientific name | Local name | Accession number | Collection |
| Synedrella nodiflora | Dudia | 37501 | National Botanical Garden, Dhaka, Bangladesh |
Extraction: The stems were collected from the national botanical garden, Dhaka, Bangladesh, during February 2010. Then the steams were washed with water and sun dried for 10 days. The dried steam was then pulverized into coarse powder in a grinding machine. 200 mg of powder was extracted in 500 ml of cold ethanol for 5 days. The solvent was filtered, squeezed off and evaporated by keeping it at room temperature and then crude extract was obtained. The percentage of yield of the crude methanol extract is given in the following Table 2.
TABLE 2: EXTRACTION OF STEM BARK OF SYNEDRELLA NODIFLORA
| Scientific name | Used part | % of yield |
| Synedrella nodiflora | stem bark | 10% |
Animal: The experiment was carried out on Swiss albino rats. The rats were aged between the 2.5-3 month of both sex and weighted between 100-105 gm (average weight 100 gm). The rats were obtained from ICDDR, b animal house of Dhaka, Bangladesh.
They were fed on standard laboratory diet were allowed to drink water. They were housed in a well-ventilated cage at room temperature in hygienic condition under natural light and dark schedule in the laboratory.
Drug and Chemicals: A drug used in the experiment were followed:
- Carbon tetrachloride (CCl4)
- The crude extract of a plant
Reagents:
- Sorbitol
- 0.9% NaCl solution
Equipments:
- Syringe with needle
- Surgical blade
- Scissor
- Forceps
- Centrifuge tube
Preparation of Sample:
Preparation of Crude Extracts (Drug) Suspension:
- In case of dose 250 mg/kg body weight of rat 500 mg of crude extract was taken and the suspension was prepared by adding with 5 ml sorbitol so that every 0.25 ml of suspension contains 25 mg of crude extract.
- In case of dose 100 mg/kg body weight of rat 200 mg of crude extract was taken and the suspension was prepared by adding with 2 ml sorbitol so that every 0.10 ml of suspension contains 10 mg of crude extract.
METHODS:
Study of Hepatoprotective Activity: The liver is especially sensitive to carbon tetrachloride. In mild cases, the liver becomes swollen and tender, and fat builds up inside the organ. In cases, liver cells may be damaged or destroyed, leading to a decreased in liver function. Carbon tetrachloride (CCl4) is frequently used as a chemical inducer for experimental liver cirrhosis. It is metabolized by the drug metabolizing enzyme of cytochrome p450 to highly reactive trichloromethyl free radical (OOCCl4).
Free radical attack membrane lipid and protein and this cause destruction of microsomes, mitochondria, and nuclei of the hepatocytes and thus the hepatocytes are damaged 12.
Induction of Hepatotoxic Rats by CCl4: Acute toxicity studies were performed according to the Organization for Economic Co-Operation and Development (OECD)-423 guidelines 13.
Hepatoprotective was induced by an intraperitoneal administration of single dose of CCl4 (3 ml/kg) which was evidenced by a significant increase of serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), ATP, Bilirubin & cholesterol 14.
Experimental Design: 15 The hepatoprotective activity of Synedrella nodiflora extract was determined by using carbon tetrachloride-induced hepatotoxic rat model. After three days of acclimatization, the rats were divided into four groups each comprising of five rats and treatment was done for 3 days.
Group 1: Normal control which received neither the sample nor CCl4.
Group 2: Control group which received only a single intraperitoneal dose of CCl4 injection.
Group 3: SN-250 group, this group consists of 5 rats, which received sample solution of crude ethanol extract of Synedrella nodiflora at a dose of 250 mg/kg and a single dose of CCl4 i.p. This group is called the experimental group.
Group 4: SN-100, this group consists of 5 rats, which received sample solution of crude extract of Synedrella nodiflora at a dose of 100 mg/kg and a single dose of CCl4 i.p. This group is called the experimental group.
Administration of the Test Samples: The suspensions of the test sample (250 mg/kg & 100 mg/kg body wt.) were administered to rats 1 h, 24 h, and 48 h after injection 16.
TABLE 3: DOSAGE REGIMEN FOR EACH GROUP OF RATS USED FOR HEPATOPROTECTIVE SCREENING
| Study group | No. of Rats | Drug/Drug administration | Dose/kg body Weight | Route of administration |
| G-1 | 5 | No drug | ------- | ----- |
| G-2 | 5 | CCl4 | 3 ml/kg | Intraperitonial |
| G-3 | 5 | CCl4 + Ethanol extract | 3 ml/kg + 250mg/kg | Intrapertonial |
| G-4 | 5 | CCl4 + Ethanol extract | 3 ml/kg + 100mg/kg | Intraperitonial |
Assessment of the Hepatoprotective Study: Four ways can assess hepatoprotective activity:
- Functional study
- Biochemical study
- Morphological study
- Histological study
In our present study, we have assessed the activity biochemically.
Biochemical Study:
Measurement of the Biochemical Parameter of Blood: After 72 h of drug treatment, blood was collected from each animal for enzyme estimation by cutting the carotid artery at the neck.
Collection of Serum From Blood: Blood sample was collected in a previously labeled centrifuging tube and allowed to clot at room temperature. Serum was separated by centrifugation of 3000 rpm for about 15 min. The cleared straw colored in vials with the help of micropipette and stored at -4 °C.
Biochemical Examination:
Parameter Studies: The following biochemical parameters were determined for the assessment of hepatoprotective activity.
- Serum Alanine Aminotransferase (ALT) or serum glutamate pyruvate transaminase (SGPT).
- Serum Aspartate Aminotransferase (AST) or serum glutamate oxaloacetate trans-aminase (SGOT).
- Serum bilirubin.
Test Principle:
Test Principle of ALT/GPT BR: Alanine aminotransferase (ALT/GPT) catalyzes the transfer of the amino group from alanine to oxoglutarate with the formation of glutamate and pyruvate. The latter is reduced to lactate by lactate dehydrogenase (LDH) in the presence of reduced nicotinamide adenine dinucleotide (NADH).
The reaction is monitored kinetically at 340 nm by the rate of decrease in absorbance resulting from the oxidation of NADH to NAD+, proportional to the activity of ALT present in the sample.
L-Alanine + 2-Oxoglutarate ALT/GPT
L-Glutamate + Pyruvate
Pyruvate + NADH + H+ LDH
Lactate + NAD+
This test has been formulated according the standarized method described by IFCC. Clin Chem Lab Med 2002; 40(7): 718-724
Test Principle of AST/GOT BR: Aspartate aminotransferase (AST/GOT) catalyzes the transfer of the amino group from aspartate to oxoglutarate with the formation of glutamate and oxaloacetate. The latter is reduced to malate by malate dehydrogenase (MDH) in the presence of reduced nicotinamide adenine dinucleotide (NADH).
The reaction is monitored kinetically at 340 nm by the rate of decrease in absorbance resulting from the oxidation of NADH to NAD+, proportional to the activity of AST present in the sample.
9-oxoglutarate + L-aspartate GOT
L-glutamate + Oxaloacetate
Oxaloacetate + NADH + H+ MDH
L-malate + NAD+
This test has been formulated according to the standardized method described by IFCC. Clin Chem Lab Med 2002; 40(7): 718-724.
Test Principle of Bilirubin: Bilirubin is converted to colored azobilirubin by diazotized sulfanilic acid and is measured photometrically. Of the two bilirubin fractions in serum –bilirubin-glucuronide and free bilirubin which is bound to albumin– only the former reacts directly, while free albumin reacts after being displaced from protein by an accelerator. The difference of two measurements total bilirubin (with accelerator), and direct bilirubin (without accelerator) enables the calculation of indirect bilirubin. The terms «direct» and «indirect» bilirubin refers exclusively to the reaction characteristics in the presence or absence of an accelerator or solubilizer and is only approximate equivalents of the two bilirubin fractions.
RESULTS AND DISCUSSION:
Biochemical Results: The biochemical parameters of the experimental group (G-3 and G-4) were compared with CCl4 treated group (G-2). A comparison was also made between the normal untreated group (G-1), and the CCl4 treated group (G-2). The change in the biochemical parameters among the different group was tabulated and tested statistically to demonstrate the significance of the study.
Effects of Acute Administration of Carbon Tetrachloride (CCl4) on Serum Transaminases (ALT, AST), Bilirubin Level in Rats: Here, a single intraperitoneal dose of CCl4 (3 ml/kg body weight) was given to the standard group. The following observations were made:
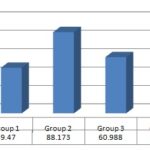
І. Serum Alanine Aminotransferase Level (ALT): Drug treatment produced an increase in serum ALT level in CCl4 treated group. The mean value of serum ALT levels in control and CCl4 treated group was 49.47 ± 2.376, 88.173 ± 5.46381 U/L respectively. The increase in serum ALT level in CCl4 treated group was highly significant as compared to the control group, which did not take CCl4. The results are shown in Table 4.
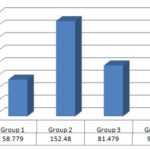
ІІ. Serum aspartate aminotransferase level (AST): The mean value of serum AST levels in control and CCl4 treated group was 58.779 ± 0.0947, 152.48 ± 0.0948 U/L respectively. The increase in serum AST level as compared to the control group was highly significant. The results are shown in Table 4.
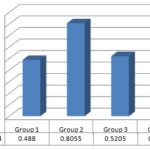
ІІІ. Serum Bilirubin Level: The mean value of serum bilirubin levels in control and CCl4 treated group was 0.488 ± 0.024495, 0.8055 ± 0.020082 respectively. The increase in serum bilirubin level as compared to the control group was highly significant. The results are shown in Table 4.
TABLE 4: SHOWING THE EFFECTS OF (CCL4) ON SERUM TRANSAMINASE (AST AND ALT), BILIRUBIN LEVEL IN RATS
| Group | No. of rats | Treatment | Mean serum ALT/SGPT level (U/L) ± SEM | Mean serum AST/ SGOT level (U/L) ± SEM | Mean serum bilirubin level (mg/dl) ± SEM | ||
| 1 | 5 | Normal | 49.47 ± 2.376 | 58.779 ± 0.0947 | 0.488 ± 0.024495 | ||
| 2 | 5 | Control | 88.173 ± 5.46381 | 151.32 ± 0.094713 | 0.8055 ± 0.02082 | ||
Control = Normal food and water only; Standard = Single dose of CCl4 3ml/kg body weight i.p; SEM = Standard error mean.
↑ = Increase in mean serum level.
Effects of CCl4 and CCl4 Plus Crude Ethanolic Extract of Synedrella nodiflora on Serum Transaminase (ALT and AST), Bilirubin Levels in Rats: Here, a single intraperitoneal dose of CCl4 (3 ml/kg body weight) was given along with the suspension of ethanol extract (250 mg/kg and 100 mg/kg body wt.). The following observations were recorded.
І. Serum Alanine Aminotransferase level (ALT) The mean value of serum ALT levels Fig. 1 in CCl4 treated groups were 88.173 ± 5.46381 and CCl4 plus crude ethanolic extract treated groups at a different dose (250 mg/kg and 100 mg/kg body wt.) were 60.988 ± 0.7936 and 73.757 ± 1.2970 respectively. The decrease in serum ALT level in ethanolic extract treated group at a 250 mg/kg and 100 mg/kg body wt. was significant compared to CCl4 treated group. The results are shown in Table 5.
ІІ. Serum Aspartate Aminotransferase level (AST): Drug treatment produced a decreased in serum AST level Fig. 2 in CCl4 plus crude ethanolic extract treated group. The mean value of serum AST levels in CCl4 and CCl4 plus crude ethanolic extract treated group at different doses of (250 mg/kg and 100 mg/kg body wt.) were 152.48 ± 0.094 and 81.479 ± 2.85039, 99.81 ± 4.98894 respectively. The decrease in serum AST level in CCl4 plus ethanolic extract treated group at a 100 mg/kg body wt. was significant compared to CCl4 treated group. The results are shown in Table 5.
ІІІ. Serum Bilirubin Level: Drug treatment produced a decrease in serum Bilirubin level Fig. 3 in CCl4 plus crude ethanolic extract treated group. The mean value of serum Bilirubin levels in CCl4 and CCl4 plus crude extract treated group at different doses (250 mg/kg and 100 mg/kg body wt.) were 0.8055 ± 0.02082 and 0.5205 ± 0.0249, 0.6385 ± 0.0237 respectively. The decrease in serum bilirubin level in CCl4 plus ethanolic extract treated group at a 100 was significant compared to the treated group. The results are shown in Table 5.
TABLE 5: EFFECT OF CCl4 AND CCl4 PLUS CRUDE ETHANOLIC EXTRACT OF SYNEDRELLA NODIFLORA ON SERUM TRANSAMINASES (ALT, AST), BILIRUBIN LEVELS IN RATS
| Group | No. of rats | Treatment | Mean serum AST/SGOT level(U/L) ± SEM | Mean serum ALT/SGPT level(U/L) ± SEM | Mean serum Bilirubin level (mg/dl) ± SEM |
| 1 | 5 | No drug | 58.779 ± 0.9479 | 49.47 ± 2.376 | 0.488 ± .024495 |
| 2 | 5 | CCl4 | 152.48 ± 0.947136 | 88.173 ± 5.46381 | 0.8055 ± 0.02082 |
| 3 | 5 | CCl4+ethanol extract | 81.479 ± 2.85039 | 60.918 ± 0.7936 | 0.5205 ± 0.0249 |
| 4 | 5 | CCl4+ethanol extract | 99.81 ± 4.98894 | 73.757 ± 1.2970 | 0.6385 ± 0.02327 |
Statistical analysis was conducted through one way ANOVA and post hoc Dunnett's test.
The CCl4 = single dose of CCl4, 3ml/kg body wt.
Ethanol extract (G-3) = Thrice dose of 250mg/kg body wt i.p.
Ethanol extract (G-4) = Thrice dose of 100mg/kg body wt i.p.
↑ = Increase in mean serum level.
↓ = Decrease in mean serum level.
Graphical Presentation of Biochemical Parameters of Tested Groups:
FIG. 1: COMPARISON OF SGPT LEVEL WITH DIFFERENT GROUP
Where, G-1 = Control or untreated group, G-2 = Standard or CCl4 treated group, G-3 = Thrice dose of ethanol extract 250 mg/kg body wt. i.p, G-4 = Thrice dose of ethanol extract 100 mg/kg body wt. i.p.
FIG. 2: COMPARISON OF SGOT LEVEL WITH DIFFERENT GROUP
Where, G-1 = Control or untreated group, G-2 = Standard or CCl4 treated group, G-3 = Thrice dose of ethanol extract 250 mg/kg body wt. i.p., G-4 =Thrice dose of ethanol extract 100 mg/kg body wt. i.p.
FIG. 3: COMPARISON OF SERUM BILIRUBIN LEVEL WITH DIFFERENT GROUPS
Where, G-1 = Control or untreated group, G-2= Standard or CCl4 treated group, G-3 = Thrice dose of ethanol extract 250 mg/kg body wt. i.p., G-4 = Thrice dose of ethanol extract 100 mg/kg body wt. i.p.
DISCUSSION: Literature review revealed that various chemical and biological investigations were carried out with this plant. Liver damage induced by CCl4 is a commonly used model for the screening of hepatoprotective drugs. The rise in the serum level of ALT, AST and bilirubin has been attributed to the damaged structural integrity of the liver, because they are cytoplasmic in location and released into circulation after cellular damages.
When rats were treated with carbon tetrachloride it induces hepatotoxicity by metabolic activation, therefore it selectively causes toxicity in liver cells maintaining semi-normal metabolic function. Carbon tetrachloride is metabolically activated by cytochrome p-450 dependent mixed oxidase in the endoplasmic reticulum to from trichloromethyl free radical (CCl3) which combined with cellular lipids and proteins in the presence of oxygen to induce lipid per-oxidation. These result in a change of structure of the endoplasmic reticulum and another membrane, loss of metabolic enzyme activation, reduction of protein synthesis and loss of glucose-6-phosphatase activation, leading to liver injury.
In the pharmaceutical study, in-vivo hepato-protective effect of Syndrella nodiflora extract had been investigated against carbon tetrachloride-induced rats at 250 mg/kg body wt. and 100 mg/kg body wt. normal rat group showed no changes in their behavior and in all biochemical parameter Table 4.2.
In carbon tetrachloride treated group it was observed that a significant increased of blood serum levels of SGPT (88.173 ± 5.46381, p<0.05), SGOT (151.32 ± 0.0947, p<0.05) and bilirubin (0.8055 ± 0.02082, p<0.05) compare to normal rats Table 4.1. For an experimental group, the plant extract was administered at a dose 250 and 100 mg/kg body wt. for three days after administration of carbon tetrachloride (3 ml/kg body wt.) at the first day. After 72 h all experimental rats were sacrificed and various biochemical parameters were determined.
Treatment with Syndrella nodiflora ethanol stem bark extract recovered the injured liver to normal after 72 h at a dose of 250 and 100 mg/kg body wt, which indicate that Syndrella nodiflora has antihepatotoxicity effect. The highest hepato-protective activity was observed for ethanol extract of Syndrella nodiflora at a dose of 250 mg/kg body wt. and the reduction of serum level of ALT, AST and serum bilirubin were 60.918 ± 0.7936, 81.479 ± 2.85039 and 0.5205 ± 0.0249 respectively. A decrease in serum bilirubin after treatment with the extract in the liver damage model indicated the effectiveness of the extract in the normal functional status of the liver 16.
The literature review also revealed that methanol extract of Syndrella nodiflora stem bark has significant hepatoprotective activity against Diclofenac-induced hepatotoxicity 17. The present biochemical analysis of said extract also showed a significant hepatoprotective activity in carbon tetrachloride-damaged liver cells.
CONCLUSION: From the overall result of the biochemical parameters, it could be inferred that ethanolic stem bark of Synedrella nodiflora showed the significant hepatoprotective activity, but appropriate effective concentration needs to be fixed. Further study on this plant could be extended for the isolation and structure determination of the hepatoprotective principle or principles.
ACKNOWLEDGEMENT: Authors are grateful to Dr. Mamunur Rashid, Professor & Chairman Department of Pharmacy, and Southeast University for providing necessary facilities to carry out this work. I comment on behalf of humanity to those rats that give their supreme sacrifice for the palliation of human sufferings.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Bataller R and Brenner DA: Liver fibrosis. J Clin Invest 2005; 115: 209-18.
- Chattopadhyay RR and Bhattacharyya SK: Terminalia chebula: An update, Pharmacog 2007; 1(1): 439-45.
- Hikino H and Kiso Y: Natural products for liver diseases. Economic and Medicinal Plant Research. Academic Press, London 1988; 2: 39‐67.
- Roy SD, Das S, Shil D and Dutta KN: Herbal hepatoprotective agents: A review.World J Pharma Res 2012; 1(2): 87-99.
- Ismail RSA, El-Megeid AAA and Aly Abdel-Moemin R: Carbon tetrachloride-induced liver disease in rats: The potential effect of supplement oils with vitamins E and C on the nutritional status. GMS German Med Sci 2009; 7. ISSN 1612-3174. Doc05. PMCID: PMC2716554
- Bhogaonkar PY, Dagawal MJ and Ghorpade D: S2 9. Pharmacological studies and antimicrobial activity of Synedrella nodiflora Bioscience Discovery 2011; 2(3): 317-321.
- Wijaya S, Nee TK, Jin KT, Hon LK, San LH and Wiart C: Antibacterial and antioxidant activities of Synedrella nodiflora (L.) Gaertn. (Asteraceae). J Complement Integr Med 2011; 8(1). doi: 10.2202/1553-3840.1499
- Forestier AM, Monforte MT, Ragusa S, Trovato A and Iauk L: Antiinflammatory, analgesic and antipyretic activity in rodents of plant extracts used in African medicine. Phytotherapy Research 1996; 10(2): 100-106,
- Bhogaonkar PY, Dagawal MJ and Ghorpade D: S2 9. Pharmacological studies and antimicrobial activity of Synedrella nodiflora. Bioscience Discovery 2011; 2(3): 317-321.
- Nahar L and Haque A: Antidiarrhoeal and hypoglycemic effects of Synedrella nodiflora. Research Gate, Phyto-pharmacology 2012; 2(2): 257-264.
- Anthea M, Hopkins J, Mclaughlin CW, Johnson S, Warner MQ, LaHart D and Wright JD: Human Biology and Health, Englewood Cliffs, New Jersey, USA 1993.
- Verma N, Singh AP and Rao CV: Protective effect of ethyl acetate fraction of Rhododendron arboreum flowers against carbon tetrachloride-induced hepatotoxicity in experimental models. Indian J Pharmacol 2011; 43(3): 291-295.
- Ecobichon DJ: New York: CRC Press. The Basis of Toxicology Testing 1997; 43-86.
- Hepatoprotective properties of Crepis rueppellii and Anisotes trisulcus: Two traditional medicinal plants of Yemen Journal of Ethnopharmacology 1986; 16(1): 105-111.
- Park EJ: Protective effect of (S)-Bakuchiol from Psoralea corylifolia on rat liver injury in-vitro and in-vivo. Planta Med 2005; 71(6): 508-513.
- Gayatri G: Hepatoprotective activity of ethanolic extract of Stachytarpheta indica on wister rats. Pharmacie Globale (IJCP) 2011; 1(04).
- Tanna A, Nair R and Chandra S: The Japan Society of Pharmacognosy Springer 23 September 2008.
How to cite this article:
Kabir T and Hossain MR: Hepatoprotective activity of ethanolic extract from the stem bark of Synedrella nodiflora on carbon tetrachloride induced hepatotoxicity in Swiss albino rats. Int J Pharmacognosy 2014; 1(5): 327-35. doi: 10.13040/IJPSR.0975-8232.1(5).327-35.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
8
327-335
620
2105
English
IJP
T. Kabir * and M. R. Hossain
Department of Pharmacy, University of Asia Pacific, Dhanmondi, Dhaka, Bangladesh.
tasmiatul.kabir@gmail.com
17 March 2014
15 April 2014
30 April 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(5).327-35
01 May 2014