GREEN SYNTHESIS, CHARACTERISATION AND ANTIBACTERIAL ACTIVITY EVALUATION OF SILVER SULFIDE NANOPARTICLES OF SENNA OCCIDENTALIS (L.)
HTML Full TextGREEN SYNTHESIS, CHARACTERISATION AND ANTIBACTERIAL ACTIVITY EVALUATION OF SILVER SULFIDE NANOPARTICLES OF SENNA OCCIDENTALIS (L.)
Victor OgwekpeEgbeneje *, Atoo Gabriel Hungwa, Shiriki Dooshima, Samuel E. Okhale, IsaccOminyi Ogbogo and Omolade Ojo
Department of Chemistry, Benue State University, P. M. B 102119, Makurdi, KM 1, Gboko Road, Benue State, Nigeria.
ABSTRACT: The study focuses on the green synthesis of silver sulfide nanoparticles using aqueous Senna occidentalis (L.) extract as reducing, capping, and stabilizing agents. Fourier Transform Infra-red Spectroscopy (FTIR) and Scanning electron microscopy (SEM) characterized the synthesized silver sulfide nanoparticles. FT-IR spectrum of Senna occidentalis leaves aqueous extract as shown in Fig. 3 below shows a number of peaks, thus reflecting its complex nature. The bands at 3175cm-1 is characteristic of the alcohol/phenol –OH stretching vibration, 2361.44cm-1 an attribute O=C= Stretching representing carbon dioxide. The weak peak at 1594cm-1 is assigned for C=O bending, indicating carboxylic acid, and the band at 995cm-1 represents the C=C bending signifying alkene. Fig. 4 below shows the FTIR result of the synthesized silver sulfide nanoparticle at different bands. The bands at 3785 and 3703 cm-1 correspond to O-H stretching vibration, indicating the presence of alcohol/phenol; the peak at 3406 cm-1 reflects N-H stretching of alphatic primary amine. Bands at 2919 and 2855.82cm-1 correspond to the C-H stretching of aromatic compounds. The band at 1713.88cm-1 indicates C=N stretching attributed to amine, the band at 1601cm-1 is assigned to C=C, a characteristic of conjugated alkene. The shape of the silver sulfide nanoparticles is spherical with few exceptional as ellipsoidal. Senna occidentalis Ag2S nanoparticles were observed to possess antimicrobial activity as it inhibited some of the tested microorganisms (bacteria and fungi), giving clear inhibition zones that were the same or higher in some cases to those of the standard antibiotic or antifungal used. The nanoparticles antifungal inhibition activity was seen to be better than griseofulvin (the standard antifungal used) in the case of Aspergillus fumigatis.
Keywords: Green, Nanoparticles, Anti microbial and characterization
INTRODUCTION: Metallic nanomaterials have been used in a wide-ranging applications in various fields. Specifically, shapes, size, and composition are significantly linked to their physical, chemical, and optical properties.
Technologies based on nanoscale materials have been exploited in various fields, from chemistry to medicine 1-3. Silver sulfide (Ag2S) nanomaterial is an important material used in various areas of studies, including antimicrobial activity 4, 5.
The synthesis of silver sulfide nanoparticles (Ag2SNPs) using several different methods had been reported, such as template-based method at room temperature and ambient pressure 6, water-in-CO2 micro emulsions 7, a microwave-assisted template-free method 8, modified homogeneous precipitation route 9, sonochemical synthesis 10, hydrothermal method 11, by multi-solvent thermal decomposition method 12, via a one-pot method in ethylene glycol with 3-mercaptopropionic acid 13, modified chemical bath deposition technique 14, synthesis of silver sulfide nanoparticles capped with either chitosan, green tea, Combtretum molle or black wattle extracts 15, chemical method 16, multi-solvent thermal decomposition method 17. All the methods and routes by which silver sulfide nanoparticles are synthesized are highly disadvantageous due to toxic chemicals used and waste products, which create environmental problems, high energy consumption, the difficulty of large-scale processes, and wasteful purifications 18-21. In recent studies, the green or benign approach in synthesizing nanomaterials has attracted significant attention to protect the environment from hazardous wastes 22. Senna occidentalis (L.), a small shrub about 3 ft. high belonging to the Leguminosae family 23, have been extensively investigated because of their rich medicinal (anti-inflammatory, anti-carcinogenic, anti-mutagenic, anti-plasmodial, anti-rheumatic and hepatoprotective) and economic uses 24–28. It is known to have been used locally in treating eczema and some other skin infections by people of Oju local area of Benue state 29. However, in an attempt to continuously search for and to bring a solution to man’s health challenges, especially microbial infections causing morbidity and mortality due to the development of resistant strains of the virus, bacteria, pathogenic fungi, and protozoa through the use of natural endowment in a more enhanced form that is eco-friendly, the objective of the present research work was to synthesize silver sulfide nanoparticles by green route using Senna occidentalis (L.) leaves aqueous extract at room temperature and study it’s antibacterial activity
MATERIALS AND METHODS:
Collection and Identification of Plant Sample: Senna occidentalis leaves were collected from Mararaba in Nasarawa states, Nigeria; the leaves were identified and authenticated at the Department of Biological Sciences, Benue State University Makurdi by a botanist Mr. Waya.
Plant Extraction: Senna occidentalis leaves were rinsed with distilled water to remove foreign matters and air dried in the chemistry laboratory Benue State University Makurdi, at room temperature for 15 days. The dried leaves were crushed and further pounded into powder using a porcelain mortar and pestle. 20 g of the powdered sample was weighed using an electronic weighing balance and transferred into an 800 mL conical flask. 200 mL of distilled water was measured using a measuring cylinder and was poured into a conical flask containing the crushed sample. The mixture was boiled for 10 minutes using a heating mantle/hot plate, after which the solution was filtered with the aid of cotton wool and a glass funnel, into a 250mL conical flask to obtain an aqueous extract kept for further analysis.
FIG. 1: SENNA OCCIDENTALIS LEAVES AND THEIR AQUEOUS EXTRACT
Green Synthesis of Silver Sulfide (Ag2SNPs): Silver sulfide nanoparticles were synthesized by mixing silver nitrate with Senna occidentalis aqueous extract and sodium sulfide at ambient temperature. In this synthesis, 1g of silver nitrate was dissolved in 100 ml of Senna occidentalis aqueous extract under magnetic stirring at 27°C (room temperature) for 10 min.
After that, a sodium sulfide solution was prepared by dissolving 1g of Sodium Sulfide in 100 mL of distilled water and gently swirling for homogeneity. The sodium sulfide solution was then added dropwise to the solution of AgNO3, and Senna occidentalis extract under continuous magnetic stirring until the colour of the solution changed to a suspended gray-black color indicating the formation of silver sulfide nanoparticles is shown below 30. The mixture was centrifuged at 8000rpm for 4 hours, the supernatant was taken out, and the residue was washed twice with 10mL of distilled water. The obtained nanoparticles were further transferred to a sample bottle for further analysis.
FIG. 2: AQUEOUS EXTRACT OF SENNA OCCIDENTALIS AND SYNTHESIZED AG2SNPS (LEFT TO RIGHT)
Characterization of Silver Sulfide Nanoparticles:
Fourier Transform Infrared Spectroscopy (FT-IR): Fourier transforms infrared spectroscopy (FT-IR) analysis was performed in all samples isolated to have a prompt result regarding the biomineral. A few crystals were mixed with KBr (Merck for spectroscopy) and pulverized in an agate mortar to form a homogenous powder from which the appropriate pellet was prepared under a pressure of 7 tons. All spectra were recorded from 4000 to 400 cm1 using the Pelkin Elmer 3000 MX spectrometer. Scans were 32 per spectrum with a resolution of 4 cm1. The IR spectra were analyzed using the spectroscopic software Win-IR Pro Version 3.0 with a peak sensitivity of 2cm1.
Scanning Electron Microscopy: Samples were coated with a platinum coating of electrically conducting material, deposited on the sample either by low-vacuum sputter coating or by high-vacuum evaporation. To examine the morphology of the synthesized Ag2S Nanoparticles.
Antimicrobial Assay:
Source of Microorganisms: Pure isolates of bacteria: Staphylococcus aureus, Pseudomonas aeruginosa, Klebseilla spp, Escherichia coli; fungi: Aspergillus niger, Aspergillus flavus, Aspergillus fumigates, Candida albicans, maintained in the microbiological laboratory of Benue State University, Makurdi were received and used for the assay.
Culture Media Preparation, Inoculation and Sensitivity Testing: Nutrient agar (Titan Biotech Ltd TMG 341) and Potato dextrose agar (HKM HCM050) were prepared according to manufacturer’s instructions following the general procedure for culture media preparation by 31. Antibiotic (chloramphenicol capsule) was added to potato dextrose agar (PDA) to inhibit bacteria growth and allow only the seeded fungal growth.
All prepared media was aseptically poured into sterile plastic petri dishes and allowed to set. The plates were subjected to a sterility test for 24 hours; only sterile plates were used for the analysis. The plates were properly labelled with codes representing the various organisms in duplicate. The bacteria were individually streaked with a wire loop uniformly on the nutrient agar plates. A suspension of the fungi spores was prepared by washing the surfaces of each fungi plate with sterile water into a sterile sample bottle. The spores in suspension were individually streaked uniformly on the potato dextrose agar plates using a sterile wire loop. The inoculated plates were incubated for 2 hours. Paper disc of 5 mm each was prepared by punching Whatman No 1 filter paper. The paper disc was separated into labelled containers for samples and control and then sterilized in an oven (UNISCOPE LAB OVEN SM 9053) at 170 °C for 30 min. They were saturated with synthesized Senna occidentalis Ag2S nanoparticles, standard antibiotic (Ciprofloxacin 500 mg) or antifungal (Greosefulvin); respectively. The disc 3 per plate was placed at equidistance on the surfaces of the labelled inoculated and pre - incubated plates following the method of 32. The plates were incubated for 24 hours and zones of inhibition where present were measured diameter-wise with the use of a transparent plastic ruler.
RESULTS AND DISCUSSION:
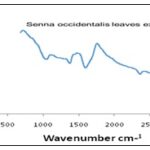
Fourier Transform Infrared Spectroscopy (FT-IR): FT-IR characterization was done to identify the types of chemical bonds (functional groups) present in aqueous extract of Senna occidentalis leaves and synthesized silver sulfide nanoparticles. FT-IR spectrum of Senna occidentalis leaves aqueous extract highlighted in Fig. 3 below shows a number of peaks, thus reflecting its complex nature.
FIG. 3: FT-IR SPECTRUM OF AQUEOUS SENNA OCCIDENTALIS
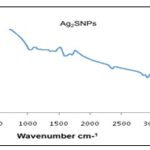
The bands at 3175cm-1 is characteristic of the alcohol/phenol –OH stretching vibration, 2361.44cm-1 an attribute of O=C= stretching, representing carbon dioxide. The weak peak at 1594cm-1 is assigned for C=O bending, indicating carboxylic acid and the band at 995cm-1 represents the C=C bending signifying alkene 33. Fig. 4 below shows the functional groups present in the synthesized silver sulfide nanoparticle. The bands at 3785 and 3703 cm-1 correspond to O-H stretching vibration, indicating the presence of alcohol/phenol, the peak at 3406 cm-1 reflects N-H stretching of aliphatic primary amine. Bands at 2919 and 2855.82cm-1 correspond to the C-H stretching of aromatic compounds. The band at 1713.88cm-1 indicates C=N stretching attributed to amine, the band at 1601cm-1 is assigned to C=C a characteristic of conjugated alkene. These functional groups play an important role as stabilizing / capping agents in synthesizing silver sulfide nanoparticles. Also, for the fingerprint, bands at 1372 and 1038 cm-1 signify S=O stretch indicating sulfonamide and sulfoxide, respectively while the band at 1280 indicates C-O stretching representing aromatic esters 34.
FIG. 4: FT-IR SPECTRUM OF SYNTHESIZED AG2SNPs
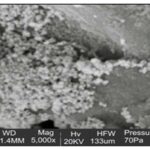
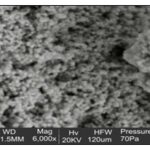
Scanning Electron Microscopy (SEM): The shape of the silver sulfide nanoparticles is spherical with few exceptional as ellipsoidal Fig. 5 and 6.
The larger silver sulfide particles may be due to the aggregation of the smaller ones due to the SEM measurements.
FIG. 5: SCANNING ELECTRON MICROSCOPY (SEM) OF SYNTHESIZED AG2SNPs OF 50UM AT A MAGNIFICATION OF 5000X
FIG. 6: SCANNING ELECTRON MICROSCOPY (SEM) OF SYNTHESIZED AG2SNPS OF 100UM AT A MAGNIFICATION OF 6000X
TABLE 1: ANTIMICROBIAL ACTIVITIES OF SENNA OCCIDENTALIS EXTRACT AG2S NANO PARTICLES ON SOME MICROORGANISMS (MM)
| Miicroorganism | Disc number | Test exreact | Control |
| Staphylococcus aureus | 1 | 8.0 | 37.5 |
| 2 | 8.5 | 38.0 | |
| 3 | 7.5 | 35.0 | |
| Average | 8.0 | 36.8 | |
| Pseudomonas aeruginosa | 1 | - | 14.0 |
| 2 | - | 13.5 | |
| 3 | - | 13.0 | |
| Average | - | 13.5 | |
| Klebseilla spp | 1 | - | 30.0 |
| 2 | - | 32.5 | |
| 3 | - | 32.5 | |
| Average | - | 31.7 | |
| Escherichia coli | 1 | 8.0 | 10 |
| 2 | 8.0 | 9.5 | |
| 3 | 8.0 | 12.5 | |
| Average | 8.0 | 10.7 | |
| Aspergillus niger | 1 | 11.0 | 9.0 |
| 2 | 12.0 | 10.0 | |
| 3 | 10.5 | 9.0 | |
| Average | 11.2 | 9.3 | |
| Aspergillus flavus | 1 | 9.5 | 10 |
| 2 | 9.0 | 10 | |
| 3 | 9.0 | 10 | |
| Average | 9.2 | 10 | |
| Aspergillus fumigatis | 1 | 9.5 | - |
| 2 | 9.5 | - | |
| 3 | 9.5 | - | |
| Average | 9.5 | - | |
| Candida albicans | 1 | 8.5 | 8.0 |
| 2 | 8.0 | 9.0 | |
| 3 | 9.0 | 10.0 | |
| Average | 8.5 | 9.0 |
Antimicrobial Studies: Antimicrobial inhibition test was conducted using the synthesized Ag2S nanoparticles and it was observed to have inhibition activity against Staphylococcus aureus and Escherichia coli bacteria; however, Pseudomonas aeruginosa and Klebseilla spp were not inhibited; fungi Aspergillus niger, A. flavus, A. fumigatus and Candida albicans were inhibited giving clear zones of inhibition as shown in Table1. The sensitivity of staphylococcus aureus to standard antibiotic ciprofloxacin was higher than that recorded by the other test bacteria and the finding agrees with work done by 35. Fungi Aspergillus fumigates was inhibited by the Senna occidentalis Ag2S nanoparticles but showed no inhibition by the standard antifungal drug used as control; this agrees to the fact that antimicrobial are met with resistance from various microorganisms, this is in agreement with the findings of 36.
CONCLUSION: In recent studies, green approach to synthesizing nano materials has attracted significant attention to protect the environment from hazardous wastes. However, the importance in the use of a benign approach in the synthesis and development of nanoparticles through the fast, eco-friendly, and convenient method cannot be overemphasized. In this study, a simple, fast, eco-friendly and convenient method was used to prepare silver sulfide nanoparticles for antimicrobial activity evaluation using Senna occidentalis leaves aqueous extract. These particles are monodispersed and spherical, with few exceptionally ellipsoidal. The method enables the bioprocess with the advantage of being environmentally friendly as no chemical reagent was used. The color change occurs as a result of the reaction with the plant moiety leading to the formation of silver sulfide nanoparticles, as confirmed by SEM and FTIR results. Antimicrobial inhibition test was conducted using the synthesized Ag2S nanoparticles, and it was observed to have inhibition activity against Staphylococcus aureus and Escherichia coli bacteria; however, Pseudomonas aeruginosa and Klebseilla spp were not inhibited; fungi Aspergillus niger, A.flavus, A. fumigatus and Candida albicans were inhibited giving clear zones of inhibition as shown in Table1. The sensitivity of staphylococcus aureus to standard antibiotic ciprofloxacin was higher than that recorded by the other test bacteria and the finding agrees with work done by 35. Fungi Aspergillus fumigates were inhibited by the Senna occidentalis Ag2S nanoparticles but showed no inhibition by the standard antifungal drug used as control; this agrees that antimicrobial is met with resistance from various microorganisms; this is in agreement with the findings of 36. Senna occidentalis Ag2S nanoparticles was observed to possess antimicrobial activity as they inhibited some of the test microorganisms (bacteria and fungi), giving clear inhibition zones that were the same or higher in some cases than those of the standard antibiotic or antifungal used. The inhibition activity on bacteria was selective as it did not inhibit some of the test organisms (Pseudomonas aeruginosa and Klebseilla spp). The nano particles' antifungal inhibition activity was seen to be better than griseofulvin (the standard antifungal used) in the case of Aspergillus fumigatis.
ACKNOWLEDGEMENT: We are grateful to the university management: Benue State University, Makurdi; for providing the enabling environment for successful research. Our gratitude also goes to the staff of our great institution's Biological Sciences and Chemistry laboratories for their help and support that has brought the research to this end.
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES:
- Millstone JE, Hurst SJ, Cutler JI and Mirkin CA: Colloidal gold and silver triangular nanoprisms small 2009; 5: 646-64.
- Lee SH, Rho WY, Park SJ, Kim J, Kwon OS and Jun BH: Multifunctional self-assembled monolayers via micro contact printing and degas-driven flow guided patterning. Sci Rep 2018; 8: 16763.
- Lee SH, Sung JH and Park TH: Nanomaterial-based biosensor as an emerging tool for biomedical applications. Ann Biomed 2012; 40: 1384-1397.
- Bruhwiler D, Leigener C, Glaus S and Calzaferri G: Luminescent silver sulfide clusters. Journal of Physical Chemistry B 2002; 106: 3770-3777.
- Fakhri A, Pourmand M, Khakpour R and Behrouz S: Structural, optical, photoluminescence and antibacterial properties of copper-doped silver sulfide nanoparticles. J of Photochemistry and Photobiology B 2015; 149: 78-83.
- Xiao J, Xie Y, Tang R and Luo W: Template-based synthesis of nanoscale Ag2E (E = S, Se) dendrites. Journal of Materials Chemistry 2002; 12: 1148-1151.
- Liu JC, Raveendram P, Shervani Z and Ikushima Y: Synthesis of Ag2S quantum dots in water-in-CO2 microemulsions. Chemical Communications 2004; 22: 2582-2588.
- Su W, Li R and Xing YJ: Preparation and characterization of hollow carambola-shaped silver sulfide microspheres using a microwave-assisted template-free method. Chinese Chemical Letters 2016; 27: 451-453.
- Xaba T, Moloto MJ, Nchoe OB, Nate Z and Moloto N: Synthesis of silver sulfide nanoparticles through homogenous precipitation route and the preparation of the Ag2S-chitsoan nanocomposites for the removal of iron (II) ion from wastewater. Chalcogenide Lett 2017; 14: 337-46.
- Du N, Zhang H, Sun H and Yang D: Sonochemical synthesis of amorphous long silver sulfide nanowires. Materials Letters 2007; 61: 235–238.
- Yu C, Leng M, Liu M, Yu Y, Liu D and Wang C: Synthesis of normal and flattened rhombic dodecahedral Ag2S particles. Crystal Eng Commun 2012; 14: 3772-77.
- Sahib IKMM, Thangaraju D, Prakash N and Masuda Y: Size controlled synthesis of silver sulfide nanostructures by multi-solvent thermal decomposition method. Journal of Crystal Growth 2017; 468: 119–124.
- Kubie L, King LA, Kern ME, Murphy JR, Kattel S, Yang Q, Stecher WD and Parkinson BA: Synthesis and characterization of ultrathin silver sulfide nanoplatelets. ACS Nano 2017; 11: 8471-8477.
- Jadhav UM, Patel SN and Patil RS: Synthesis of silver sulfide nanoparticles by modified chemical route for solar cell applications. Res J of Chem Scien 2017; 3: 69-74.
- Sibiya PN and Moloto MI: Green synthesis of Ag2S nanoparticles: Effect of pH and capping agent on size and shape of NPs and their antibacterial activity. Digest J of Nanomaterials and Biostructures 2018; 13: 411-418.
- Zhao Y and Song Z: Phase transfer-based synthesis of highly stable, biocompatible and the second near-infrared-emitting silver sulfide quantum dots. Materials Letters 2014; 126: 78-80.
- Khaleelullah MMSI, Dheivasigamani T, Natarajana P, Masuda Y, Inamib W, Kawataa Y and Hayakawa Y: Size controlled synthesis of silver sulfide nanostructures by multi-solvent thermal decomposition method. Journal of Crystal Growth 2017; 468: 119-124.
- Cárdenas Riojas AA, Wong A, Planes GA, Sotomayor MDPT, La Rosa-Toro A and Baena-Moncada AM: Development of a new electrochemical sensor based on silver sulfide nanoparticles and hierarchical porous carbon modified carbon paste electrode for determination of cyanide in river water samples. Sensors and Actuators B: Chemical 2019; 287: 544-550.
- Sadovnikov SI: Thermal stability and recrystallization of semiconductor nanostructured sulfides and sulfide solid solutions. J of Alloys and Compounds 2019; 788: 586-599.
- Huang Z, He K, Song Z, Zeng G, Chen A, Yuan L, Li H and Chen G: Alleviation of heavy metal and silver nanoparticle toxicity and enhancement of their removal by hydrogen sulfide in Phanerochaete chrysosporium. Chemosphere 2019; 224: 554-561.
- Maharubin S, Zhou Y and Tan GZ: Integration of silver nanoparticles and microcurrent for water filtration. Separation and Purification Technology 2019; 212: 57-64.
- Akl M. Awwad, Nida M. Salem, Marwa M Aqarbeh and Fatin M. Abdulaziz: Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chemistry International 2020; 6(1): 42-48.
- Isah T and Mujib A: In-vitro plant regeneration of coffee senna (Senna occidentalis) from hypocotyl-derived callus. Acta Biological Cracoviensia 2013; 55(2): 120–5.
- Sharma N, Trikha P, Athar M and Raisuddin S: In-vitro inhibition of carcinogen-induced mutagenicity by Cassia occidentalis and Emblica officinalis. Drug Chem Toxicol 2000; 23: 477 84.
- Vashishtha VM, John TJ and Kumar A: Clinical and pathological features of acute toxicity due to Cassia occidentalis in vertebrates. Indian Journal of Medical Research 2009; 130: 23–30.
- Yadav JP, Arya V, Yadav S, Panghal M, Kumar S and Dhankhar S: Cassia occidentalis: A review on its ethnobotany, phytochemical and pharmacological process. Fitoterapia 2009; 81: 223–30.
- Tona L, Mesia K, Ngimbi NP, Chrimwami B, Okond’ahoka CK and de Bruyne T: In-vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasit 2001; 95: 47-57.
- Kayina A and Reddy GSN: Effect of organic manures, biofertilizers and inorganic fertilizers on growth and yield of senna (Cassia angustifoliavahl.). Life Sciences leaflets 2012; 6: 35–41. doi:http://lifesciencesleaflets.ning.com/.
- Egbeneje VO: Ethno-botanical study of selected plants use, preparation and application by people of OJU local government area of Benue state, Nigeria. Int J Pharmacognosy 2021; 8(7): 100-07.
- Awwad MA, Nida MS, Marwa MA and Fatin MA: Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. J of chemistry Intern and Scientific Organization 2020; 6(1): 42-48.
- Cheesbrough M and Cheesbrough M: District Laboratory Practice in Tropical Counties. Low Price Edition, Cambridge University Press Cambridge UK 2006; 48-49.
- Akpomie OO, Akponah E, Ehwarieme AD and Paul RE: Antimicrobial activity of coconut water, oil and palm kernel oils extracted from coconut and palm kernel on some plasmid-mediated multi-drug resistant organisms associated with food spoilage. African Journal of Microbiology Research 2020; 14(7): 366-373.
- IR Spectrum Table by Frequency Range.http://www.sigmaaldrich.com/NG/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table. Retrieved on the 01/02/2022
- Niraimathi KL, Sudha V, Lavanya R and Brindha P: Biosynthesis of silver nanoparticles using Aternanthera sessilis (Linn.) extract and their antimicrobial, antioxidants activities. Colloids and Surfaces B; Biointerfaces 2013; 102: 288-291.
- Amare A, Worku T, Ashagirie B, Adugna M, Getaneh A and Dagnew M: Bacteriological profile, antimicrobial susceptibility patterns of the isolatesamong street vended foods and hygienicpractice of vendors in Gondar town, Northwest Ethiopia: a cross sectional study. BMC Microbiology 2019; 19: 120.
- Selvamohan T, Ramadas V and Kishore SSS: Antimicrobial activity of selected medicinal plants against some selected human pathogens bacteria. Advances in Applied Science Research 2012; 3(5): 33374-3381.
How to cite this article:
Egbeneje VO, Atoo HG, Dooshima S, Okhale SE, Ogbogo IO and Ojo O: Green synthesis, characterisation and antibacterial activity evaluation of silver sulfide nanoparticles of Senna occidentalis (L.). Int J Pharmacognosy 2022; 9(6): 116-22. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.9(6).116-22.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
116-122
1239 KB
971
English
IJP
Victor OgwekpeEgbeneje *, Atoo Gabriel Hungwa, Shiriki Dooshima, Samuel E. Okhale, IsaccOminyi Ogbogo and Omolade Ojo
Department of Chemistry, Benue State University, P. M. B 102119, Makurdi, KM 1, Gboko Road, Benue State, Nigeria.
egbenejevictor2018@gmail.com
22 April 2022
24 June 2022
27 June 2022
10.13040/IJPSR.0975-8232.IJP.9(6).116-22
30 June 2022