GASTRO-PROTECTIVE ACTIVITY OF THE LEAVES AQUEOUS EXTRACT OF DIOSPYROS MESPILIFORMIS ON GASTRIC ULCERS IN SWISS MICE
HTML Full TextGASTRO-PROTECTIVE ACTIVITY OF THE LEAVES AQUEOUS EXTRACT OF DIOSPYROS MESPILIFORMIS ON GASTRIC ULCERS IN SWISS MICE
Andre Perfusion Amang * 1, Parfait Bourvoune 1, Christophe Mezui 2, Gaël Tchokomeni Siwe 3, Mesmine Teukam Kuissu 4 and Paul Vernyuytan 3
Department of Biological Sciences 1, Faculty of Science, University of Maroua, P.O. Box 814, Maroua, Cameroon.
Department of Biological Sciences 2, Higher Teachers’ Training College, University of Yaoundé I. P. O. Box 47, Yaoundé, Cameroon.
Department of Animal Biology and Physiology 3, Faculty of Science, University of Yaoundé I. P. O. Box 812, Yaoundé, Cameroon.
Institute of Medical Research and Study of Medicinal Plants 4, P. O. Box 13033 Yaoundé, Cameroon.
ABSTRACT: Information from ethnomedicine suggests that Diospyros mespiliformis possesses antiulcer properties. Thus, the purpose of this study was to evaluate its gastroprotective activity. The gastroprotective activity of the leaves aqueous extract of D. mespiliformis was evaluated by three experimental models of gastric ulcers in mice, namely: HCl/ethanol, HCl/ethanol with indomethacin pre-treatment and indomethacin (p. o). Ulcerated surface, ulcer index, and mucus mass were recorded. A qualitative phytochemical analysis of the extract was carried out. The administration of D. mespiliformis extract prevented the formation of gastric lesions against the necrotizing agents. For HCl/ethanol induction, the extract at 100, 200, and 400 mg/kg dose-dependently inhibited ulcer formation of 28.36, 29.19 and 35.82%, respectively. Indomethacin pre-treatment reduced the preventive effect of the extract to 19.69 and 28.24% at 200 and 400 mg/kg, respectively. As for indomethacin induction, the extract at 200 mg/kg showed the most important ulcer inhibition (88.13%). For all three induction models, a significant increase in mucus secretion between 44.75 and 121.34% was observed. D. mespiliformis could prevent the formation of gastric ulcers by stimulating mucus secretion via its direct action on mucus-secreting cells due to the presence of flavonoids, tannins, anthocyanins, and saponins in the extract.
| Keywords: |
Diospyros mespiliformis, Gastric ulcers, Gastro-protection, Phytoconstituents
INTRODUCTION: Gastric ulcer is a loss of substance from the gastric wall reaching deep into the muscularis 1 and is the most common disease of the gastrointestinal tract. It affects about 10% of the world population and particularly those in non-industrialized countries 2. It results from an imbalance between the aggressive and protective factors of the gastric mucosa 3.
Aggressive factors may be exogenous, including Helicobacter pylori infection, alcohol, non-steroidal anti-inflammatory drugs, and tobacco consumption 4 or endogenous, including hydro-chloric acid, pepsin and reactive oxygen species (ROS) 5. These factors often lead to a weakening of the protective factors, causing thus significant damage of the gastric mucosa, such as bleeding and especially inflammation 6. Due to the multiple etiologies of gastric ulcers, several synthetic drugs are used for their treatment, namely: histamine H2 receptor antagonists, anticholinergics, proton pump inhibitors, antacids, prostaglandin analogs, and antibiotics 7.
However, some of these drugs promote not only a wide range of adverse effects, but are also costly, making them inaccessible to most people in non-industrialized countries. So, phytotherapy seems to be a serious alternative for the management of gastric ulcers. For this purpose, several plants are used, namely: The roots of Vernonia kotschyana 8. The leaves of Eremomastax speciosa 9 the bark of the Parkia biglobosa 10 and the bark, stems and roots of Trichilia emetica 11. Diospyros mespiliformis is a plant belonging to the Ebenaceae family. In Cameroon, it is listed among medicinal plants with a wide therapeutic arsenal, namely, antipyretic, analgesic, and anti-inflammatory effects 12. On the other hand, the healing activity of D. mespiliformis leaves on wounds has been cited in ethnomedicine by Burkill 13, yet it is known that wound healing plants may have anti-ulcerogenic properties.
Thus, this study was designed to evaluate the gastro-protective activity of the leaves aqueous extract of D. mespiliformis on experimental models of gastric ulcers in mice.
MATERIALS AND METHODS:
Plant Material: The leaves of Diospyros mespiliformis were harvested in January 2018 in Mayo-Oulo in the North Region of Cameroon. They were collected with the help of a botanist (Professor Tchopsala). The plant has been authenticated at the Garoua Wildlife School by comparison with the existing specimen deposited under code HEFG/01404.
Animal Material: Male albino’s mice, of 13 to 14 weeks old, with bodyweight ranging from 25 to 30 g were used. These mice were raised at the animal house of the University of Ngaoundéré (Cameroon). They were subjected to a natural day/night cycle. The diet was constituted of 50% maize flour, 20% soya flour, 10% cotton cake, 10% fish meal, 5% beef bone, 4% vegetable oil, 1% salt, with free access to tap water.
Preparation of the Extract: The leaves of D. mespiliformis after harvesting were washed and dried in a shed at room temperature. Using an electric shredder, the dry leaves were reduced in a fine powder and then weighed using a sensitive electrical scale. 250 g of this powder were macerated in 2 liters of distilled water for 48 h. The macerate obtained was filtered using Whatman paper no. 3. The resulting filtrate was dried in an oven at a temperature of 40 °C for 24 h. The dry extract obtained had a mass of 10.5 g, with a yield of 4.2%, and was stored at 4 °C for subsequent tests.
Phytochemical Test: The leaves aqueous extract of D. mespiliformis was subjected to a qualitative phytochemical screening to detect the presence of some classes of phytoconstituents (phenols, alkaloids, flavonoids, terpenoids, sterols, tannins, glucoside, anthraquinone, coumarins, anthocyanins, saponins, and proteins) using the method described by Harborne 14.
Gastric Ulcers Tests:
HCl / Ethanol-Induced Gastric Ulcers: The induction of gastric ulcers with HCl / ethanol was carried out according to the method described by Hara and Okabe 15. 25 mice were deprived of food for 24 h before the experiment but with free access to water. These were divided into five groups of five animals, including three experimental groups which received the aqueous extract of D. mespiliformis orally (at the doses of 100, 200 and 400 mg/kg, respectively) and two control groups (positive and negative) which received orally, sucralfate (50 mg/kg) and distilled water (0.5 ml/ 30 g), respectively. After 1 h, all groups were given HCl/ethanol solution (150 mM/ 60%), and 1 h later, the mice were sacrificed. The stomachs were removed, and 5 ml of 2% formalin were injected into. The mucus from each stomach was gently scraped off by using glass slides, then the length and width of ulcers were measured, and scores assigned according to the method described by Tan et al., 16. The ulcer index (UI), percentage of inhibition (% I) and percentage of ulcerated surface (% US) were also calculated.
HCl/Ethanol-Induced Gastric Ulcers in Mice Pre-Treated with Indomethacin: HCl/ethanol-induced gastric ulcers with indomethacin pre-treatment were performed according to the model described by Sun et al., 17. The animals were divided into four groups of five mice each and fasted for 24 h before the experiment. They were given indomethacin intraperitoneally at the dose of 20 mg/kg. Thirty min later, the treated groups received extract at the doses of 200 and 400 mg/kg. The positive and negative control groups received orally sucralfate (50 mg/kg) and distilled water (0.5 ml / 30 g), respectively. One hour later, all animals were given per os HCl/ethanol (0.5 ml / 30 mg). One hour later, these animals were sacrificed and the stomachs were examined in the same manner as for the HCl/ethanol model.
Indomethacin-Induced Gastric Ulcers: Gastric mucosa ulcers were induced by the method described by Pillai and Santhakumari 18. After 24 h of fasting, the mice were divided into four groups of five mice each. The two test groups received extract (200 and 400 mg/kg, respectively) orally, while those in positive and negative control groups received sucralfate (50 mg/kg) and distilled water (0.5 ml / 30 g), respectively.
One hour later, all animals were given indomethacin solution per os at the dose of 50 mg/kg. 5 h later, the animals were sacrificed. The remaining procedure was the same as described for HCl / ethanol induction.
Statistical Analysis: The results obtained were expressed as mean ± standard error on the mean (SEM). They were statistically analyzed using one-way ANOVA (analysis of variance), and multiple comparisons between groups were performed by the Student-Newman-Keuls post-test. The software Graph Pad Prism 5 was used for statistical analysis, and the values of p<0.05 were considered as significant.
RESULTS:
Results of Phytochemical Test: Phytochemical analysis of the leaves aqueous extract of D. mespiliformis revealed the presence of the following classes of bioactive compounds: phenolic compounds, flavonoids, tannins, anthocyanins, saponins and proteins Table 1.
Antiulcer Tests:
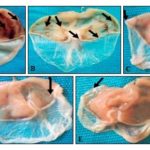
Effects of the Leaves Aqueous Extract of D. mespiliformis on HCl / Ethanol-Induced Gastric Ulcers: Intragastric administration of HCl/ethanol to mice resulted in characteristic Fig. 1 lesions on the glandular part of the stomach. These lesions are in the form of dark red bands, which are more numerous and larger size in the negative control Fig. 1A. Their size decreased by about half in the positive control Fig. 1B. The lesions size decreased more and gradually in dose-dependent (100, 200, and 400 mg/kg) manner in extract-treated groups, Fig. 1C, 1D, and 1E.
TABLE 1: RESULTS OF PHYTOCHEMICAL TEST OF THE LEAVES AQUEOUS EXTRACT OF D. MESPILIFORMIS
| Classes of compounds | Observation |
| Phenolic compounds | + |
| Alkaloids | - |
| Flavonoids | + |
| Terpenoids | - |
| Tannins | + |
| Glucosides | - |
| Anthraquinones | - |
| Coumarins | - |
| Anthocyanins | + |
| Saponins | + |
| Proteins | + |
+ = presence; - = absence
FIG. 1: PHOTOGRAPH OF STOMACHS ULCERATED WITH HCl/ETHANOL (a): Negative control, (b): Positive control, (c): 100 mg/kg of extract, (d): 200 mg/kg of extract, (e): 400 mg/kg extract dose: Indication of gastric ulcers
The leaves aqueous extract of D. mespiliformis (100, 200 and 400 mg/kg) induced a significant and dose-dependent decrease of the ulcerated area (12.60; 11.60 and 9.60 mm2, respectively) compared to the negative control (42.40 mm2); corresponding to an increase in the inhibition percentage of 28.36; 29.19 and 35.82, respectively. This inhibition of the degree of ulceration was accompanied by a significant increase of mucus secretion of 11.58; 12.34; and 16.82 mg, respectively, compared to the negative control (8.00 mg) Table 2.
TABLE 2: EFFECTS OF AQUEOUS EXTRACT OF D. MESPILIFORMIS ON HCl/ETHANOL-INDUCED GASTRIC ULCERS
| Treatment | N | Dose (mg/kg) | US (mm2) | % US | UI | % I | MM (mg) | % MM |
| Negative control | 5 | - | 42.40 ± 4.28 | 13.25 | 4.83 ± 0.41 | - | 8.00 ± 1.938 | |
| Sucralfate | 5 | 50 | 24.60 ± 3.47** | 8.31 | 3.77 ± 0.21*** | 21.95 | 9.88 ± 0.78 | 23.50 |
| D. mespiliformis
D. mespiliformis D. mespiliformis |
5 | 100 | 12.60 ± 2.79*** | 3.66 | 3.46 ± 0.22*** | 28.36 | 11.58 ± 0.65 | 44.75 |
| 5 | 200 | 11.60 ± 2.24*** | 3.65 | 3.42 ± 0.15*** | 29.19 | 12.34 ± 0.67 | 54.25 | |
| 5 | 400 | 9.60 ± 1.75*** | 3.00 | 3.10 ± 0.10*** | 35.82 | 16.82 ± 1.62** | 110.25 |
The values are expressed as mean ± SEM; ** p< 0.01; *** p<0.001; significant differences compared to the negative control using student-newman-keuls multiple comparison test followed by one-way anova; n = 5, number of mice per groups; us = ulcerated surface; % us = percentage of ulcerated surface; ui = ulcer index; %i = percentage of inhibition: mm = mucus mass; % mm = percentage mucus increase
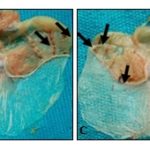
Effects of the Leaves Aqueous Extract of D. Mespiliformis on HCl / Ethanol-Induced Gastric Ulcers with Indomethacin Pre-treatment: Macroscopic observations showed, in the glandular part of the stomach, formation of gastric lesions induced by HCl/ethanol with indomethacin pre-treatment. The lesions were larger and more numerous in the negative control, and they decreased in animals treated with the extract and sucralfate Fig. 2.
FIG. 2: PHOTOGRAPHS OF STOMACHS ULCERATED WITH HCL/ETHANOL WITH INDOMETHACIN PRETREATMENT (a) Negative control; (b) Positive control; (c) Dose 200 mg/kg extract; (d) Dose 400 mg/kg extract, :Indication of gastric ulcers
Indomethacin pre-treatment reduced the preventive effects of the extract (200 and 400 mg/kg), evidenced by a decrease of the percentage of inhibition for HCl/ethanol induction with indomethacin pre-treatment (19.69 and 28.65, respectively) Table 3, compared to HCl/ethanol induction (29.19 and 35.82, respectively) Table 2. This reduction in preventive effects was accompanied by a decrease in mucus secretion at the dose of 400 mg/kg of extract from 16.82 mg to 10.46 mg after pre-treatment with indomethacin Table 2 and 3.
TABLE 3: EFFECTS OF AQUEOUS EXTRACT OF D. MESPILIFORMIS ON HCL/ETHANOL-INDUCED GASTRIC ULCERS WITH INDOMETHACIN PRETREATMENT
| Treatment | N | Dose (mg/kg) | US (mm2) | % US | UI | % I | MM (mg) | % MM |
| Negative control | 5 | - | 23.60 ± 3.17 | 7.38 | 3.86 ± 0.18 | 4,78 ± 0.68 | ||
| Sucralfate | 5 | 50 | 28.80 ± 3.80 | 9.00 | 3.46 ± 0.13 | 10.36 | 9.20 ± 0.18*** | 92.47 |
| D. mespiliformis
D. mespiliformis |
5 | 200 | 10.60 ± 1.90* | 3.31 | 3.10 ± 0.10 | 19.69 | 10.58 ± 1.93*** | 121.34 |
| 5 | 400 | 8.60 ± 2.36* | 2.69 | 2.77 ± 0.69* | 28.24 | 10.46 ± 1.81*** | 118.83 |
The values are expressed as Mean ± SEM;*p < 0.05; *** p<0.001; significant differences compared to the negative control using student-Newman-Keuls multiple comparison test followed by one-way ANOVA; N = 5, number of mice per groups; US = ulcerated surface; % US=percentage of ulcerated surface; UI = ulcer index; %I = percentage of inhibition: MM = mucus mass; % MM = percentage of mucus increase
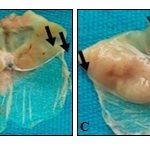
Effects of the Leaves Aqueous Extract of D. Mespiliformis on Indomethacin-Induced Gastric Ulcers: Extract at the dose of 200 mg/kg prevented ulcers formation (88.13%) induced with indomethacin, corresponding to a significant decrease of ulcerated surface (0.40 mm2) compared to negative control (11.40 mm2) Fig. 3. Extract (200 mg/kg) induced also a significant increase (p<0.001) of mucus production (10.90 mg) compared to negative control (5.50 mg) Table 4.
FIG. 3: PHOTOGRAPH OF STOMACHS ULCERATED WITH INDOMETHACIN (a) Negative control; (b) Positive control; (c) Dose 200 mg/kg extract; (d) Dose 400 mg/kg extract, indication of gastric ulcers
TABLE 4: EFFECTS OF AQUEOUS EXTRACT OF D. MESPILIFORMIS ON GASTRIC ULCERS INDOMETHACIN- INDUCED
| Treatment | N | Dose (mg/kg) | US | % US | UI | % I | MM | % MM |
| Négative control | 5 | - | 11.40 ± 3.37 | 3.56 | 3.37 ± 0.30 | 5.50 ± 0.53 | - | |
| Sucralfate | 5 | 50 | 3.00 ± 1.38** | 0.94 | 1.80 ± 0.73* | 46.59 | 6.26 ± 0.79 | 13.81% |
| D. mespiliformis
D. mespiliformis |
5 | 200 | 0.40 ± 0.40*** | 0.13 | 0.40 ± 0.40** | 88.13 | 10.90 ± 1.19** | 98.18% |
| 5 | 400 | 0.80 ± 0.80*** | 0.25 | 0.60±0.60** | 82.20 | 9.50 ± 1.83* | 80.00% |
The values are expressed as mean ± SEM; **p< 0.05;** p< 0.01; *** p<0.001; significant differences compared to the negative control using student-newman-keuls multiple comparison test followed by one-way ANOVA; N = 5, number of mice per groups; US= ulcerated surface % US = percentage of ulcerated surface UI = ulcer index; % I = percentage of inhibition: MM = mucus mass; % MM = percentage of mucus increase
DISCUSSION: Diospyros mespiliformis possesses a wide range of medicinal uses 19. Based on its numerous virtues, the study of its gastroprotective properties was investigated by using three models of gastric ulcer induction: HCl/ethanol induction, HCl/ethanol induction with indomethacin pre-treatment and indomethacin induction in mice.
Several factors, both endogenous and exogenous, are responsible for the establishment of peptic ulcers, following an imbalance between aggressive and protective factors of the gastric mucosa. Alcohol consumption and NSAID intake are among the most common and dangerous ulcerogenic factors leading to gastroduodenal damage 20. For this reason, models of ulcer lesions with HCl/ethanol and indomethacin were selected. Indomethacin is the first choice of NSAID for producing the experimental ulcers because it has the highest ulcerogenic potential compared to other NSAIDs 21. Macroscopic observations of stomachs subjected to HCl/ethanol showed characteristic gastric lesions in the glandular part of the stomach; these are in the form of dark red bands. These lesions are characteristic of the lesions induced by this mixture, similarly to the observations of studies by Anandan et al., 22, Silva et al., 23 and Martins et al., 24 which showed the necrotic power of HCl/ethanol on the stomachs of rats. The HCl/ethanol mixture acts by exerting a direct toxic effect on the epithelium, leading to the formation of characteristic necrotic lesions due to a vasoconstrictor effect on veins and arteries of the gastric mucosa, to a decrease in blood flow thus producing congestion and inflammation 25 solubilization of stomach mucus components and oxidative stress 24.
The results of this study show that the leaves aqueous extract of D. mespiliformis at 200 and 400 mg/kg significantly (p˂0,001) reduced the percentage of ulceration (3.65 and 3.00) corresponding to percentages of inhibition of 29.19 and 35.82, respectively. These results suggest that the extract protects the gastric mucosa against the ulcerogenic agent similarly to by Antonio et al., 26, who showed that the Solanum variabile extract prevented the formation of gastric lesions induced by HCl / ethanol. The leaves aqueous extract of D. mespiliformis (100, 200, and 400 mg/kg) induced a very significant (p<0.01) increase of mucus secretion (11.58, 12.34 and 16.8 mg, respectively) compared to the negative control (8 mg). Indeed, mucus constitutes a line of defense as mentioned by Pasquier 27; it is characterized by a film formed by the polymerization of glycoproteins that traps bicarbonates, to delay the penetration of endo-luminous H+ ions and thus establish a pH gradient ranging from less than 3 at the luminal surface of this layer, to more than 7 on the mucous surface.
To determine the cytoprotective mechanism of action by which the leaves aqueous extract of D. mespiliformis acts to increase mucus secretion, the HCl/ethanol induction with indomethacin pre-treatment was performed. Mice pre-treated with indomethacin showed ulcers similar to those obtained with HCl / ethanol. Indomethacin is a non-steroidal anti-inflammatory drug (NSAID) that inhibits the secretion of endogenous prostaglandins. These prostaglandins protect the stomach against damage by stimulating bicarbonate and mucus secretion, maintaining gastric microcirculation, and regulating stomach mucosal repair 28, 29.
The inhibitory action of indomethacin exposes the gastric mucosa, thus allowing the HCl/ethanol mixture to attack the gastric wall and generate ulcers. This would explain the severity of the lesions observed on the mucosa of mice subjected to pre-treatment with indomethacin. A decrease in the ulcerated surface was observed in the extract-treated groups at the doses of 200 (10.60 mm2) and 400 mg/kg (8.60 mm2) compared to the negative control (23.60 mm2).
The extract (200 and 400 mg/kg) also increased mucus secretion (10.58 and 10.46 mg, respectively) compared to the negative control group (4.78 mg). These results are in line with those obtained by Mezui et al., 30 who had shown that the increase in mucus secretion by the aqueous extract of Cassia arereh following HCl/ethanol with indomethacin pre-treatment model was not related at the action of endogenous prostaglandins; which could be the case of our extract. To verify the gastro-protective mechanism of action of the leaves aqueous extract of D. mespiliformis, the induction method with indomethacin was performed. The main mechanism by which indomethacin induces gastric ulcers implies inhibition of the biosynthesis of endogenous prostaglandins by inhibiting cyclo-oxygenase.
Indeed, indomethacin causes non-selective inhibition of cyclo-oxygenase 1 and cyclo-oxygenase 2, which are enzymes responsible of the synthesis of prostaglandins. Prostaglandins play an important cytoprotective role in the gastric mucosa by positively influencing mucus and bicarbonate secretion, surface epithelial cells and mucosal circulation 31, 32. Their absence leads to mucosal damage resulting in gastric ulcers 33, 34. Analysis of our results shows that treatment of mice with aqueous extract of D. mespiliformis leaves (200 and 400 mg/kg) significantly inhibited ulcer formation (88.13 and 82.20%) associated with a significant increase in mucus secretion (10.90 and 9.50 mg) compared to the negative control (5.50 mg). These results suggest that the extract may have a direct action on mucus-secreting cells.
The observed anti-ulcer activity of the extract could be attributed to its richness in classes of bioactive compounds that would strengthen the muco-bicarbonate barrier. Phytochemical tests revealed the presence of several classes of bioactive compounds such as flavonoids, tannins, saponins and anthocyanins. These results are similar to those obtained by Adeniyi et al., 35 as classes of bioactive compounds revealed by the qualitative phytochemical analysis were the same. Several studies have demonstrated the anti-ulcerogenic effect of tannin-rich plants. Gege-Adebayo et al., 36 associated the anti-ulcerogenic potential of Ocimum gratissimum with its high tannin content. Indeed, tannins prevent the formation of ulcers due to their ability to precipitate proteins forming a protective layer, thus, preventing the action of aggressive factors on the gastric mucosa.
Their vasoconstrictive and astringent action on the site of the ulcer forms an impermeable layer on the wall and thus prevents the formation of gastric ulcers 37. As for the flavonoids, several mechanisms have been proposed to explain their gastroprotective effects, including enhancement of prostaglandin secretion in the gastric mucosa and decrease of histamine secretion by mast cells through inhibition of histidine decarboxylase 38.
This anti-ulcer activity might also be due to anthocyanins activity, as Alvarez-Suarez et al., 39 have shown that anthocyanins increase GSH levels and antioxidant enzyme activity. For saponins, their protective activities are linked to the fact that they stimulate mucus production factors (prosta-glandins) in the mucosa.
CONCLUSION: At the end of this work, it appears that the leaves aqueous extract of Diospyros mespiliformis protected the gastric mucosa against the damage induced by various necrotizing agents. The gastro-protective action could be attributed to the increase in mucus secretion by a mechanism that is not linked to the action of endogenous prostaglandins but rather to the direct stimulation of mucus secretory cells, due of the presence of flavonoids, tannins, anthocyanins and saponins in this extract.
ACKNOWLEDGEMENT: The present work has been financed by author’s personal funds.
CONFLICTS OF INTEREST: Authors declare no conflict of interest.
REFERENCES:
- Guyton H: Textbook of Medical Physiology, Saunders Elsevier, Edition 12th 2011; 1112.
- Diniz PBF, Ribeiro ARS, Estevama CH, Bani CC and Thomazzi SM: Possible mechanisms of action of Caesalpinia pyramidal against ethanol-induced gastric damage. Journal of Ethno Pharmacology 2015; 168: 79-86.
- Nanjundaraje UA, Narula P and Thomson M: Peptic ulcer disease. Pediatrics and Child Health 2014; 24: 485-90.
- Al-Wajeeh NS, Hajrezaei M, Al-Henhena N, Kamran S, Bagheri E, Zahedifard M, Saremi K, Noor SM, Ali HM and Abdulla MA: The antiulcer effect of Cibotium barometz leaves in rats with experimentally induced acute gastric ulcer. Drug Design Development and Therapy 2017; 11: 995-09.
- Rozza AL, Hiruma-Lima CA, Tanimoto A and Pellizzon CH: Morphologic and pharmacological investigations in the epicatechin gastro protective effect. Evidence Based Complementary and Alternative Medicine 2012; 1-8.
- Almeida ESDS, Filho VC, Niero R, Clasen BK, Balogun SO and Martins DTDO: Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea St-Hil. in animal models. Journal of Ethno Pharmacology 2011; 134: 630-36.
- Bastista LM, Lima GR, De-Almeida AB, Magri LP, Calvo TR, Ferreira AL, Pellizzon CH, Hiruma-Lima CA, Vilegas W, Sano PT and Brito A: Ulcer healing and mechanisms of action involved in the gastro protective activity of fractions obtained from syngonanthus arthrotrichus and syngonanthus bisulcatus. BMC Complementary and Alternative Medicine 2015; 15(391): 1-9.
- Sanogo R, De-Pasquale R, Germanò MP, Iauk L and De-Tommasi N: Vernonia kotschyana bip. tolerability and gastro protective activity. Phytotherapy Research 1996; 10: 169-71.
- Amang AP, Mezui C, Siwe TG, Zondengoumba NE, Enoh EG and Tan PV: Prophylacticand healing activities of the leaves aqueous extract of Eremomastax speciosaon gastric ulcers in rats. Journal of Advances in Biology and Biotechnology 2017; 12: 1-13.
- Aklikokou AK, Gbeassor M and Napo K: Action anti-ulcéreuse de quelques plantes médicinales. Pharmacopée ET Médecine Traditionnel Africaine 1995; 55-60.
- Diallo D: Ethno Pharmacological survey of medicinal plants in Mali and phytochemical study of four of them: Glinus oppositifolius (Aizoaceae), Diospyros abyssinica (Ebenaceae), Entada Africana (Mimosaceae), Trichilia emetic (Meliaceae), thèse doctorat. Université de Lausanne Suisse 2000; 221.
- Adzu B, Ben AC, Florence DT, Oluwakanyinsola AS and Ogbaji JI: Isolation and analgesic property of lupeol from Diospyros mespiliformis stem bark. Journal of Medicinal Plants Research 2015; 9: 813-19.
- Burkill H: The useful plant of west Tropical Africa. Royal Botanic Gardens Kew, London, Volume 1985; 1: 610-11.
- Harborne JB: Textbook of phytochemical methods. a guide to modern techniques of plant analysis. Chapman and Hall Ltd London Edition 5th 1998; 21-72.
- Hara N and Okabe S: Effect of generate on acute lesion in rats. Folio Pharmacologia Japonica 1985; 85: 443-48.
- Tan PV, Nditafon GN, Yewah MP, Ayafor JF and Dimo T: Eremomastax speciosa: Effect on the leaf aqueous extract on ulcer formation and gastric secretion in rats. Journal of Ethno Pharmacology 1996; 54: 139-42.
- Sun Z, Matsumoto T and Yamada H: Antiulcer activity and mode of action of the polysaccharide fraction from the leaves Panax ginseng. Planta Medical 1992; 58: 432-35.
- Pillai N and Kumari SG: Effects of nimbi din on acute and chronic gastro duodenal ulcer models in experimental animals. Planta Medi 1984; 50: 143-47.
- Etkin NL: Antimalarial plants used by Hausa in Northern Nigeria. Tropical Doctor 1997; 27: 12-16.
- Adhikary B, Yadav KS, Bandyopadhyay SKR and Chattopadhyay S: Black tea and theaflavins assist healing of indomethacin-induced gastric ulceration inmice by antioxidative action. Evidence-Based Complementary and Alternative Medicin 2010; 1-11.
- Suleyman H, Albayrak A, Bilici M, Cadirci E and Halici Z: Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 2010; 33(4): 224-34.
- Anandan R, Rekha DR, Saravanan N and Devaki T: Protective effects of Picrorrhiza kurroa against HCl/ethanol-induced ulceration in rats. Fitoterapia 1999; 70: 498-01.
- Silva JS, Andreo MA, Tubaldini FR, Varanda EA, Rocha LRM, Brito ARMS, Vilegas W and Hiruma-Lima CA: Differences in gastro protective and mutagenic actions between polar and apolar extracts of Ananas ananassoides. Journal of Medicinal Food 2008; 11(1): 160-68.
- Martins JLR, Rodrigues ORL, Silva DM, Galdino PM, De-Paula JR, Romão W, Costa HB, Vaz BG, Ghedini PC and Costa EA: Mechanisms involved in the gastro protective activity of Celtis iguan aea (Jacq). Sargent on gastric lesions in mice. Journal of Ethno Pharmacology 2014; 155: 1616-24.
- Boligon AA, Freitas RB, Brum TF, Waczuk EP, Klimaczewski CV, De-Ávila DS, Athayde ML and Bauermann LF: Antiulcerogenic activity of Scutia buxifoliaon gastric ulcers induced by ethanol in rats. Acta Pharmaceutica Sinica B 2014; 4: 358-67.
- Antonio JM, Gracioso JS, Toma W, Lopez LC, Oliveira F and Souza-Brito ARM: Antiulcerogenic activity of ethanol extract of Solanum variabile (false “jurubeba”). Journal of Ethno Pharmacology 2004; 93: 83-88.
- Pasquier C: Stress oxydatif ET inflammation. Revue Française Des Laboratoires 1995; 276: 87-91.
- Hiruma-Lima CA, Calvo TR, Rodriguez CM, Andrade FDP, Vilegas W and Brito ARM: Antiulcerogenic activity of Alchornea castaneae folia effects on somatostatin, gastrin and prostaglandin. Journal of Ethno Pharmacology 2006; 104: 215-24.
- Okokon JE, Antia BS and Emem EE: Antiulcerogenic activity of ethanolic leaf extract of Lasianthera africana. African Journal of Traditional Compl and Alternative Medicines 2009; 6: 150-54.
- Mezui C, Nkenfou C, Amang AP, Ndji OG, Nkwengoua E, Djougné P, Lémoupa B, Fouman JM and Tan PV: Gastro protective, antioxidant and antibacterial properties of the aqueous root bark extract of Cassia arerehdel. (caesalpiniaceae) in a Wistar rat model. Journal of Advances in Biology and Biotechnology 2017; 12: 1-13.
- Dey I, Lejeune M and Chadee K: Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. British Journal of Pharmacology 2006; 149: 611-23.
- Wallace JL: Prostaglandins, NSAIDs and gastric mucosal protection: why doesn’t the stomach digest itself. Physiological Revue 2008; 88: 1547-65.
- Gehan HH, Magdy KAH and Rauuia SA: Gastro protective effect of simvastatin against indomethacin-induced gastric ulcer in rats: role of nitric oxide and prostaglandins. European Journal of Pharmacology 2009; 607: 188-93.
- Belli N, Mesbah L, Chebab S, Tekouk M and Leghouchi E: Stressoxydant induit par la co-exposition au plomb ET au cadmium: deux contaminants deseaux souterraines d’oued Nil (Jijel - Algérie). Journal of Water Science 2010; 23(3): 289-01.
- Adeniyi BA, Odetola HA and Oso BA: Antimicrobial potentials of Diospyros mespiliformis (Ebenaceae). African Journal of Medicine and Medical Science 1996; 25: 221-24.
- Gege-Adebayo GI, Igbokwe VU, Shafe MO, Akintayo CO and Mbaka DI: Anti-ulcer effect of Ocimum gratissimum on indomethacin induced ulcer and percentage of superoxide dismutase on Wistar rats. Journal of Medicine and Medical Sciences 2013; 4: 8-12.
- Agbaje EO and Okpara CS: Antiulcer activity of aqueous extract of fresh leaf of Brassica oleraceaevar. acephala (D.C) alef (Brassicaceae). International Research Journal of Pharmacy 2013; 4: 107-11.
- Sumbul S, Aftab-Ahmad M, Asif M and Akhtar M: Role of phenoliccompounds in peptic ulcer: an overview. Journal of Pharmacy and Bioallied Sciences 2011; 3: 361-67.
- Alvarez-Suarez JM, Dekanski D, Ristić S, Radonjić NV, Petronijević ND, Giampieri F, Astolfi P, González-Paramás AM, Santos-Buelga C, Tulipani S, Quiles JL, Mezzetti B and Battino M: Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. Public Library of Science One 2011; 6(10): 25-31.
How to cite this article:
Amang AP, Bourvoune P, Mezui C, Siwe GT, Kuissu MT and Vernyuytan P: Gastro-protective activity of the leaves aqueous extract of Diospyros mespiliformis on gastric ulcers in swiss mice. Int J Pharmacognosy 2020; 7(2): 100-08. doi link: http://dx.doi.org/10.13040/ IJPSR.0975-8232.IJP.7(2).44-51.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
44-51
465
994
English
IJP
A. P. Amang *, P. Bourvoune, C. Mezui, G. T. Siwe, M. T. Kuissu and P. Vernyuytan
Department of Biological Sciences, Faculty of Science, University of Maroua, Maroua, Cameroon.
perfusionamang@yahoo.fr
15 January 2020
22 February 2020
26 February 2020
10.13040/IJPSR.0975-8232.IJP.7(2).44-51
29 February 2020