EXTRACTION AND CHARACTERIZATION OF STARCH FROM THE TUBERS OF ANTIGONON LEPTOPUS SPECIES
HTML Full TextEXTRACTION AND CHARACTERIZATION OF STARCH FROM THE TUBERS OF ANTIGONON LEPTOPUS SPECIES
A. T. Hemke *, N. D. Sangle, K. K. Chandak, A. R. Pounikar and M. J. Umekar
Department of Pharmaceutical Chemistry, Smt. Kishoritai Bhoyar College of Pharmacy, New Kamptee, Nagpur - 441002, Maharashtra, India.
ABSTRACT: Starch is a natural polymer and is a group of polysaccharides composed of glucopyranose units joined together by glycosidic linkages. Starch is an important polysaccharide extensively used in many industries like food, cosmetics, paper, textile, pharmaceutical, etc. It is found to be present in appreciable quantity in stem, roots, leaves, seeds, fruits, and tubers. Hence this study was conducted to isolate and characterize starch from the tuber of Antigonon leptopus species. In this work, starch from Antigonon leptopus tubers was extracted, and % starch yield was calculated as well as evaluated for its macroscopic characters, Preliminary phytochemical screening of aqueous extract for the presence of carbohydrate, tannins, saponins, and flavonoids. The result showed that there was a sufficient amount of starch yield. The compound microscope showed the presence of round and oval-shaped small and large starch particles, single as well as in groups. The diamond and prism-shaped calcium oxalate crystals were also seen. Thus, starch of tuber Antigonon leptopus species shows good properties and could serve as alternatives for the production of industrial products that may require starch.
| Keywords: |
Starch, Antigonon leptopus tuber, Microscopic characterization, phytochemical screening
INTRODUCTION: Starch is one type of carbohydrate reserve in various plants part such as in leaves, flowers, fruits, and different types of stems and roots. In a plant, starch is used as a source of carbon and energy. Starch made up of glucose residues that are linked in two different forms, such as glucose moiety link with two polymers Amylase (with α-1, 4 linkages) and amylopectin (joined by α-1, 6 linkages). Starch mainly comprises of 70-80% of Amyl pectin, whereas amylose consists of 15-30% of starch 1. Acts as glucoside reserve of plants found in maize, wheat, and potato from which it is extracted, as well as in many other plants: rice, barley, vegetables, manioc, and sweet potato.
Starch is synthesis in plants via the photosynthesis process, and this mechanism is utilized by plants to produce and store the glucose (elementary sugar), which is necessary for their growth and reproduction 2. The leaves of Antigonon leptopus was evaluated for its pharmacognostic evaluation, and it showed the presence of both simple and compound form of starch grains 3. The various parts of Antigonon leptopus (family Polygonaceae), like tubers and flowers of it, consumed as food in several parts of the world. Tea preparation of aerial portions such as flowers used as a cold remedy 4, 5.
The pharmacological action behind its use of functional food qualities and it was found that the methanol extract of the aerial parts of A. leptopus, inhibited lipid peroxidation (LPO) by 89% and cyclooxygenase enzymes, COX-1 and COX-2 by 50.4% and 72.5%, respectively, at 250 lg/ml. The extracted and purified methanolic extract of Antigonon leptopus yields n-hentriacontane, ferulic acid, 4-hydroxycinnamic acid, quercetin-3-rham-noside, and kaempherol-3-glucoside along with b-sitosterol, b-sitosterol-glucoside, and d-mannitol and hence shows as an antibacterial, anti-inlamatory, anti-oxidant activity, etc.6 Also, it has been used to treat diabetes, asthma, liver and spleen disorders, cough and throat constriction, flu-related pains, hypertension, antithrombin agent, and used to reduced menstrual pains 7-10. A literature survey revealed that the extract of the plant was evaluated for structural characterization, antioxidant and anticancer properties of gold nanoparticles of extract, 11 for new steroidal saponin 12, as well as for extermination of fish bacterial pathogens 13. Being one of the tropical sources of starch that has not been utilized for industrial applications is Antigonon leptopus tuber. However, the extraction of starch has not been reported previously in the literature; hence the present study is designed with the objective to study the extraction of starch from tubers Antigonon leptopus species and characterization of starch using microscopic and preliminary phytochemical screening for potential industrial applications.
MATERIALS AND METHODS:
Sample Collection and Preparation: Fresh and healthy tuber was collected from near places in Kamptee, Durga Society-new Yerkheda, and identified by the Botany department of Nagpur. The fresh tubers were dried, pulverized, and powdered for starch extraction.
Starch Extraction: 100 mg of fresh tubers were collected, thoroughly washed with distilled water, cut into small pieces. Then these pieces were grinded in an electrical mixture to get a fine slurry. It was then filtered through a muslin cloth. It was allowed to settle overnight, then the liquid decanted and settled residue was then washed with again the distilled water and allowed to settle for a few hours after this, the water was decanted, and the residue was allowed to dry. % yield was calculated, and its morphological, microscopic characteristics were evaluated. The identification of chemical consti-tuents was confirmed by means of chemical tests.
Starch Yield: Starch yield was measured in percentage by comparing the weight of obtained starch (dry basis) with the weight of dry matter sample (Antigonon leptopus tuber). The Starch Yield (SY) was determined by the equation:
SY % = W1 / W2 × 100
Where W1 is the weight of dried starch, and W2 is the weight of the original sample (Antigonon leptopus tuber). Starch Granules Microscopic Evaluation (Morphology) Smear of isolated starch powder as well as smear stained with dilute Iodine solution was prepared on a glass slide and observed under a compound microscope.
Preliminary Phytochemical Screening: 14 Tubers of Antigonon leptopus were dried pulverized to a coarse powder, and aqueous extract was prepared by maceration. It was filtered and subjected to Preliminary phytochemical screening.
Tests for Carbohydrates:
Molish’s Test: To 3 ml of the test solution, two drops of alcoholic solution of α-Naphthol were added. The mixture was shaken well, and few drops of concentrated sulphuric acid were added slowly along the sides of the test tube. A violet ring at the junction of two lights indicates the presence of carbohydrates.
- Benedict’s Test: To 3 ml of the test solution, 3 ml of Benedict’s reagent was added. The mixture was heated on a boiling water bath for 2 min. A characteristic colored precipitate indicates the presence of sugar.
- Fehling’s Test: 1 ml of Fehling’s A solution and 1ml of Fehling’s B solution were mixed, boiled for 1 minute, an equal amount of test solution was added to it. The reaction mixture was heated in a boiling water bath for 5-10 minutes. First, yellow, then red precipitate of cuprous oxide indicates the presence of sugar.
- Iodine Test: 3 ml of test solution was mixed with a few drops of dilute Iodine solution Formation of deep blue color indicates the presence of starch. It disappears on boiling and reappears on cooling.
Tests for Proteins:
Millon’s Test: To 3 ml of the test solution, few drops of Millon’s reagent were added. A white precipitate indicates the presence of proteins.
1. Biuret Test: To 3 ml test solution, 2ml Biuret reagent was added, violet color indicates the presence of proteins.
Test with Trichloroacetic Acid: To 3 ml of test solution, few drops of trichloroacetic acid were added; the appearance of a precipitate indicates the presence of proteins.
Xanthoproetic Test: To 3 ml of the test solution, 1ml of concentrated nitric acid was added and boiled, the yellow precipitate is formed. After cooling it, 40% sodium hydroxide solution added; orange color indicates the presence of proteins.
Lead Acetate Test: To 3 ml the test solution, few drops of lead acetate solution were added. The appearance of a yellow color precipitate indicates the presence of proteins.
Tests for Amino Acids:
Ninhydrin Test: To 3 ml of the test solution, 2 ml of ninhydrin solution was added. The appearance of purple color indicates the presence of amino acids.
Millon’s Test: To 3 ml test solution, about 2 ml of Millions reagent was added, a white precipitate indicates the presence of amino acids.
Tests for Fixed oils and Fats:
Spot Test: A small quantity of powder was pressed between two filter papers. Oil stain on the paper indicates the presence of fixed oils.
Tests for Alkaloids:
Mayer’s Test: To 3 ml of the test solution, few drops of Mayer’s reagent were added. The appearance of a creamy white precipitate indicates the presence of alkaloids.
Wagner’s Test: To 3 ml of test solution, few drops of Wagner’s reagent were added. A reddish-brown precipitate indicates the presence of alkaloids.
Dragendorff’s Test: To 3 ml test solution, few drops of Dragendorff’s reagent were added. The formation of a reddish-brown precipitate indicates the presence of alkaloids.
Hager’s Test: To 3 ml test solution, few drops of Hager’s reagent were added. The formation of a yellow-orange precipitate indicates the presence of alkaloids.
Tests for Glycosides:
Anthraquinone Glycosides:
Borntrager’s Test: To 3 ml of the test solution, dilute hydrochloric acid was added; it was then boiled and filtered. Then an equal volume of chloroform was added and shaken, the chloroform layer was separated, and an equal volume of dilute ammonia solution was added to it. The appearance of pink or red color indicates the presence of anthraquinone glycosides.
Modified Borntrager’s Test: 5 ml of the test solution, 5 ml of 5% aqueous ferric chloride solution, and 5 ml of dil. HCl was heated for 5 min in a boiling water bath, cooled, and shaken with an equal volume of chloroform. The chloroform layer was separated, and 10% ammonia solution was added to it. The appearance of pink or red color indicates the presence of C type of Anthraquinone glycosides.
- Cardiac Glycosides:
Keller-Killiani Test ( for Deoxy-Sugars): To 2 ml of the test solution, few drops of glacial acetic acid and 1 drop of 5% ferric chloride were added, and the contents were then transferred to a small test tube, 0.5 ml of concentrated sulphuric acid was added carefully by the side of the test tube. A reddish-brown color appears at the junction of two liquids layer, and the upper layer becomes bluish-green; it indicates the presence of deoxy-sugar.
Raymond’s Test: The test solution was treated with hot methanolic alkali; violet color indicates the presence of cardiac glycoside.
Legal’s Test: The test solution was treated with 1ml of pyridine and 1 ml of sodium nitroprusside solution, Pink to blood red color indicates the presence of cardiac glycoside.
Baljet’s Test: The 2 ml of test solution was treated with sodium picrate; the formation of orange color indicates the presence of cardiac glycoside.
- Coumarin Glycosides: Drugs containing coumarins possess Aromatic odor. A small quantity of test solution was placed in a test tube and was covered with filter paper moistened with dilute sodium hydroxide solution. The covered test tube was placed on a water bath for several minutes, the paper was removed and exposed to ultraviolet (UV) light, presence of green fluorescence indicates the presence of Coumarin glycosides. Alcoholic test solution, when made alkaline, shows blue or green fluorescence.
Saponin Glycosides:
Froth Formation Test: 3 ml of test solution was shaken well with water in a test tube; the formation of stable froth (foam) indicates the presence of saponin glycoside.
Haemolysis Test: To 3 ml test solution, 1 drop of blood was added and allowed to stand for 15 min, settling down of RBCs indicates the presence of saponin.
Tests for Phenols and Tannins:
Ferric Chloride Test: 3 ml test solution was treated with 5 % ferric chloride solution, the appearance of blue color indicates the presence of hydrolyzable tannins, and the appearance of green color indicates the presence of condensed tannins
Phenazone Test: To 5 ml of the test solution, 0.5 gm to sodium acid phosphate was added, it was warmed and filtered. To the filtrate, 2% Phenazone solution was added, bulky precipitate indicates the presence of tannin.
Lead Acetate Test: To 3 ml of the aqueous test solution, few drops of lead acetate solution were added, the formation of a precipitate indicates the presence of tannins.
Potassium Dichromate Test: To the 3 ml of the test solution, few drops of potassium dichromate solution were added, the appearance of a red precipitate indicates the presence of tannin.
Tests for Steroids:
Liebermann-Burchard Test: To 3ml of the test solution, few drops of acetic anhydride were added, boiled, and cooled. Then concentrated sulphuric acid was added from the side of the test tube, the brown ring was formed at the junction two layers, and the upper layer turns green, which shows the presence of steroids and the formation of deep red color indicates the presence of triterpenoids.
Salkowski test: To 3 ml of the test solution, chloroform was added and concentrated sulphuric acid was added from the side of the test tube; the chloroform layer shows red to blue color and the acid layer shows greenish-yellow fluorescence.
Tests for Flavonoids: Shinoda Test: To 3 ml of the test solution, 5 ml of 95% ethanol, a few drops of conc. hydrochloric acid and 0.5 g magnesium turnings were added, pink scarlet, crimson red, or occasionally green to blue color appears after a few minutes, which indicates the presence of flavonoids.
Alkaline Reagent Test: To the test solution, few drops of sodium hydroxide solution were added, there is the formation of intense yellow colour which becomes colorless on addition of few drops of dilute acid indicates presence of flavonoids.
Lead Acetate Test: To 5ml of test solution, few drops of lead acetate solution were added. Yellow precipitate indicates presence of flavonoids.
Test for Terpenoids: 1 ml of test solution was treated with 1% CuSO4 solution; formation of green colour indicates the presence of Diterpene.
Test for Gum and Mucilage: Few ml of test solution was dissolved in 10 ml of distilled water and to this 2 ml of absolute alcohol was added with constant stirring. White or cloudy precipitate indicates the presence of Gums and Mucilage.
RESULTS AND DISCUSSION:
Macroscopic Characterization: The fresh tubers of A. leptopus were evaluated for its macroscopic as well as microscopic characters.
TABLE 1: MACROSCOPIC CHARACTERISTICS OF ANTIGONON LEPTOPUS TUBERS
| S. no. | Property | Observation |
| 1 | Colour | Whitish pink |
| 2 | Texture | Slippery |
| 3 | Odor | Slight |
| 4 | Taste | Bitter and areca nut like |
As literature survey revealed that the tuberous plants contain starch in a sufficient amount and Antigonon leptopus is one of the tuberous plants hence its tuber is rich in starch. The starch was extracted from Antigonon leptopus tuber, then it was evaluated for its macroscopic evaluation, and % starch yield was calculated.
TABLE 2: MACROSCOPIC CHARACTERISTICS OF ISOLATED STARCH OF ANTIGONON LEPTOPUS TUBERS
| S. no. | Property | Observation |
| 1 | Colour | White |
| 2 | Consistency | Solid |
| 3 | Odour | Odourless |
| 4 | Taste | Tasteless |
| 5 | % Yield | 4.73 % w/w |
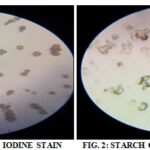
Microscopic Characteristics of Isolated Starch of A. Leptopus Tubers: Smear of isolated starch powder was prepared on a glass slide and observed under compound microscope showed the presence of round shaped small and large starch grains, single as well as in groups. The diamond and prism shaped calcium oxalate crystals were also seen. Smear of isolated starch powder was prepared on a glass slide was, stained with dilute Iodine solution and observed under compound microscope ,showed the presence of dark bluish black colour, round shaped small and large starch grains, single as well as in groups. The diamond and prism-shaped calcium oxalate crystals were also seen in Fig. 3-6.
STARCH GRAINS AND CALCIUM OXALATE CRYSTALS STAINED WITH IODINE SOLUTION SHOWN IN FIG. 3-6.
Confirmation of Presence of Starch: The presence of starch is confirmed by performing an iodine test on a glass slide. The observation is as follows.
TABLE 3: CHEMICAL TEST OF STARCH WITH IODINE SOLUTION:
| Test | Observation | Inference |
| Iodine + starch grains in a powder | Blue color observed | Starch is present |
Preliminary Phytochemical Screening: The aqueous extract was prepared by maceration. It was filtered and subjected to Preliminary phytochemical screening.
TABLE 4: PRELIMINARY PHYTOCHEMICAL SCREENING
| Phyto-constituents | Name of Tests | Result | ||
| Alkaloids
|
i) Mayers reagent test
ii) Wagners reagent test iii) Dragendorff’s Test iv) Hagers test |
-Ve
-Ve -Ve -V |
||
| Glycosides
|
i) Brontrager test
ii) Killer killani test iii) Legal test iv) Baljet test |
-Ve
-Ve -Ve |
||
| Tannins and phenolic compounds | i) Lead acetate test
ii) Ferric chloride test iii) Gelatin test |
+Ve
+Ve +Ve |
||
| Flavonoids
|
i) Shinoda test
ii) Lead acetate test iii) Alkaline reagent test |
+Ve
+Ve +Ve |
||
| Saponins | i) Froth formation test | +Ve | ||
| Carbohydrates | i) Molisch reagent test
ii) Benedict test iii) Fehling reagent test |
+Ve
+Ve +Ve |
||
FIG. 7: ANTIGONON LEPTOPUS STARCH
CONCLUSION: From the present studies, it may be concluded that the quantity of starch isolated is very less as compared to the quantity isolated from other sources of starch. However, the tubers showed the presence of carbohydrates, tannins, saponins, and flavonoids, so these compounds can be isolated and evaluated for their therapeutic potential and can be formulated into a suitable dosage form.
ACKNOWLEDGEMENT: Nil
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Alcázar-Alay SC and Meireles MA: Physicochemical properties, modifications and applications of starches from different botanical sources. Food Science and Technology 2015; 35(2): 215-36.
- Carvalho JF: 7-Starch: Major sources, properties and applications as thermoplastic materials. Handbook of Biopolymers and Biodegradable Plastics 2012; 129-52.
- Deshpande A and Joshi AB: Microscopic Studies and Physicochemical Evaluation of Antigonon Leptopus Leaves 2016; 1(6): 11-14.
- Mulabagal V, Alexander-Lindo RL, DeWitt DL and Nair MG: Health-beneficial phenolic aldehyde in Antigonon leptopus Evidence-Based Complementary and Alternative Medicine 2011.
- Vanisree M, Ruby LAL and Muraleedharan GN: Health-beneficial phenolic aldehyde in Antigonon leptopus tea. Evidence Based Complementary and Alternative Medicine 2010.
- Mulabagal V, Alexander-Lindo RL, DeWitt DL and Nair MG: Functional Food Components of Antigonon Leptopus Food Chemistry 2008; 106: 487-92.
- Lans CA: Ethnomediciene used in Trinidad and Tobago for urinary problems and diabetes mellitus. Journal of Ethnobiology and Ethnomedicine 2006; 2(1): 11-14.
- Idu M and Onyibe HI: Medicinal Plants of Edo State, Nigeria. Research Journal of Medicinal Plant 2007; 1 (2): 32-41.
- Mitchell SA and Ahmad MH: A Review of Medicinal Plants Research at the University of the West Indies, Jamaica. West Indian Med J 2006; 55 (4): 243-69.
- Chistokhodova C, Nguyen T, Calvin I, Kachirskaia G, Cunningham and Miles HD: Antithrombin activity of medicinal plants from central florida. J Ethanopharmacol 2002; 18: 277-80.
- Balasubramani G, Ramkumar R, Krishnaveni N, Pazhanimuthu A, Natarajan T and Sowmiya R: Perumal. Structural characterization, antioxidant and anticancer properties of gold nanoparticles synthesized from leaf extract (decoction) of antigonon leptopus hook & arn. Journal of Trace Elements in Medicine and Biology 2015; 30: 83-89.
- Apaya MK, Chichioco-Hernandez CL. New Steroidal Saponin from Antigonon Leptopus Hook. and Arn. Pharmacognosy Magazine 2014; 10(3): 501.
- Balasubramani G, Deepak P, Sowmiya R, Ramkumar R and Perumal P: Antigonon leptopus: a potent biological source for extermination of fish bacterial pathogens providencia and aeromonas. Natural Product Research 2015; 29(10): 958-60.
- Khandelvar KR: Practical pharmacognosy techniques and experiments. Nirali Prakashan 2008; 149-50.
How to cite this article:
Hemke AT, Sangle ND, Chandak KK, Pounikar AR and Umekar MJ: Extraction and characterization of starch from the tubers of Antigonon leptopus species. Int J Pharmacognosy 2020; 7(1): 328-33. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(11).328-33.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
10
328-333
756
1143
English
IJP
A. T. Hemke *, N. D. Sangle, K. K. Chandak, A. R. Pounikar and M. J. Umekar
Department of Pharmaceutical Chemistry, Smt. Kishoritai Bhoyar College of Pharmacy, New Kamptee, Nagpur, Maharashtra, India.
atulhemke321@gmail.com
10 June 2020
23 September 2020
28 September 2020
10.13040/IJPSR.0975-8232.IJP.7(11).328-33
01 November 2020