EVALUATION OF METHANOLIC EXTRACT OF CLITORIA TERNATEA HEPATO-PROTECTIVE AND NEPHROPROTECTIVE ACTIVITY IN RATS
HTML Full TextEVALUATION OF METHANOLIC EXTRACT OF CLITORIA TERNATEA HEPATO-PROTECTIVE AND NEPHROPROTECTIVE ACTIVITY IN RATS
Srikanta Chandra * 1, Avik Das 2, Tathagata Roy 3, Preeta Bose 3, Lucky Mukherjee 4 and Jyotirmay Samanta 5
Department of Pharmacology 1, Jakir Hossain Institute of Pharmacy, Raghunathganj, Murshidabad - 742225, West Bengal, India.
Department of Pharmacology 2, Gupta College of Technological Sciences, Asansol, West Burdwan - 713301, West Bengal, India.
Department of Pharmaceutical Technology 3, JIS University, Kolkata - 700109, West Bengal, India.
Bengal College of Pharmaceutical Science & Research 4, Durgapur, West Burdwan - 713212, West Bengal, India.
Genex College of Pharmaceutical Science & Technology 5, Chinsurah, Hooghly - 712102, West Bengal, India.
ABSTRACT: Objective: The aim of the study was to investigate the nephroprotective and hepatoprotective activity of methanolic extract of Clitoria ternatea in cisplatin and CCl4 induced in rats. Methods: Methanolic extract of aerial part of Clitoria ternatea plant was studied for its nephroprotective and hepatoprotective activity in animal experimental models. Nephrotoxicity was induced by cystone 16 mg/kg b.w. The standard drug was taken silymarin. The test drug was given methanolic extract Clitoria ternatea 500 mg/kg, 1000 mg/kg. Hepatoxicity was induced by CCl4. The standard drug was taken cisplatin 100 mg/kg. Test drugs were given extract of Clitoria ternatea 500 mg/kg and 1000 mg/kg as per b.w. Results: In hepatoprotective activity positive control group was provided with CCl4 and increased SGPT, SGOT, ALP level compare to negative control group whereas test 2 group was provided with methanolic extract of Clitoria ternatea 1000 mg/kg decreased SGPT, SGOT, ALP level compare to standard group. In nephroprotective activity positive control group was provided with CCl4 increased urea and creatinine level whereas test 2 group are provided with methanolic extract of Clitoria ternatea 1000 mg/kg decreased urea and creatinine level. Conclusion: On evaluating biochemical parameters it was found that methanolic extract of Clitoria ternatea 1000 mg/kg showed hepatoprotective and nephroprotective activity in rats.
| Keywords: |
SGPT, SGOT, ALP, Nephroprotective, Hepatoprotective
INTRODUCTION: The Liver is among the most complex and pivotal organs in the human body; it lays below the diaphragm in the abdominal pelvic region of the abdomen.
The Liver is a reddish-brown organ with four lobes of unequal size and shape. It is both largest internal organ & largest gland in the human body. It is connected to two large blood vessels one is called hepatic artery, and another one is called portal vein.
It constituents about 2.5% of an adult’s body weight. It produced bile, an alkaline compound which aids in digestion via the emulsification of lipids. Two major types of cells populate the liver lobes one is parenchyma and another one is non parenchyma cells. Sinusoidal endothelial cells, kuffer cells & hepatic stellate cells are some of the non parenchyma cells that line hepatic sinusoid. The liver plays a pivotal role in regulating various physiological processes. It is also involved in several vital functions, such as metabolism, secretion, and storage. It has great capacity to detoxicate toxic substances and synthesize useful principles. It helps in the maintenance, performance and regulating homeostasis of the body. It involved almost all the biochemical pathways to growth, fight against disease, nutrient supply, energy provision, and reproduction. It aids the metabolism of carbohydrate, protein and fat detoxification, secretion of bile, and storage of vitamins. Chemicals that cause liver injury are called hepatotoxins 1.
Certain medicinal agents, when taken in overdose & sometimes even when introduced within therapeutic ranges, may injure the organ. Other chemical agents, such as those used in laboratories (e.g. CCl4, Paracetamol) and industries (e.g.: Lead, arsenic), natural chemicals (e.g.: microcystins, aflatoxins) and IFN, herbal remedies (Cascara Sagrada, ephedra) can also induce for hepatotoxicity. Chemicals these agents are converting in chemically reactive metabolites in Liver, which have the ability to interconnect with cellular macro-molecules namely protein, lipids and nucleic acids, leading to protein dysfunction, lipid peroxidation, DNA damage, and oxidative stress. This damage of cellular function can dismiss in cell death & likely liver failure 2. In the modern treatment strategy having some limitation. Silymarin is associated with nausea, vomiting, headache. Hence we found a good rationale beyond probing for the hepatoprotective and nephro-protective activity in our pipeline drug that is Clitoria ternatea 3.
MATERIALS & METHOD:
Animal Husbandry & Statutory Approval: Healthy male and female rats (Wistar albino) age of 4-8 weeks were selected after physical and behavioral veterinary examination from Institutional Animal Ethics Committee of Gupta College of Technological Sciences, Asansol. The weight range fell within ± 20% of the mean body for each sex at the time of initiation of treatment. All experiments involving animals complies with the ethical standards of animal handling and approved by IAEC. All the selected animals were kept under acclimatization on the same day. The animal will be acclimatized for minimum 5 days before initiation of dosing. The rats were housed in standard polypropylene cages with stainless steel top grill in group of 6 rats per cage. Clean autoclaved paddy husk was used as bedding. The paddy husk was changed at least thrice in a week.
The animals were kept in a clean environment with 12 h light & 12 h dark cycles. The air was conditioned at 22 ± 30 °C and the relative humidity was maintained between 55-65% with 100% exhaust. Standard rat pellet feed was provided ad libitum throughout the study, except overnight fasting prior to blood collection & was offered the feed immediately after completion of blood collection of all the animals. Drinking water was provided ad libitum in polypropylene bottles with a stainless steel slipper tube throughout study period 4.
Collection of Plant: Leaves of Clitoria ternatea were collected from Rampurhat, West Bengal, India in August 2018 ad was authenticated by the Head of Botany Department of Government College, Rampurhat, Birbhum, West Bengal, India.
Authentication: A herbarium sheet was prepared and it was sent to head of Botany Department of Government College, Rampurhat, Birbhum, West Bengal, for authentication no of study plant is Ref No: Rph/BOT/2018/42.
Extraction: Extraction may be defined as the treatment of the plant or animal tissues with solvent, whereby the medicinally active constituents are dissolve (menstrum) and most of the inert matter remains undissolved (marc). The effective extraction of leaves depends largely on solubility and functional group consideration 5.
Soxhlet Extraction: Soxhlet extraction is used where a small volume of hot menstruum is passed over the drug time and again to dissolve out the active constituents until the drug is exhausted. This process is known as soxhlation. The Soxhlet apparatus required for the hot percolation is made from a very high grade of glass and consists three parts (a) a flask in which the menstruum is boiled (b) an extracting chamber in which drug is filled, is fitted with the side tube and a siphon (c) a condenser. The drug to be extracted, in suitably comminuted from was unusual packed in a thimble made of filter paper which was then placed into the wider part of extractor. The thimble was used to prevent chocking of the lower part of extraction by drug particles. Menstruum was placed into the flask and boiled, the vapor was allowed to pass through the side tube to the condenser where they are condensed and fall on the packed drug, through which it extracts out the active constituents. As the volume of menstruum in the extractor increase, the level of liquid in the siphoned out into flask. On further heating the menstruum vaporizes while they dissolve active constituent remains behind in the flask.
The alternate filling of the emptying of the body of the extractor goes on continuously till the drug was exhausted. Thus, the same quantity of menstruum was made to Soxhlet repeatedly, about 14 - 15 times through the drug, and the active constituents were collected in flask. This process is not suitable for the drugs containing thermolabile active constituents 6.
Successive Solvent Extraction: After the selection, collection and drying of the leaf of Clitoria ternate Linn. extraction was done. In pharmacy the solvent used for extraction purposes is known as menstruum and residue left after extracting the desired constituent is known as marc. The effective extraction of plant materials depends largely on solubility and functional group consideration.
The powder rhizomes were subjected to cold maceration and successive Soxhlet extraction using various solvent of increasing polarity namely petroleum ether, chloroform, acetone, and methanol Each time before extracting with next solvent powdered was dried in an air oven below 50 °C The extract was concentrated by distilling off the solvent and then evaporating to dryness on water -bath 7.
Extractive Value: The method determines the amount of active constituent in a given amount of medicinal plant material when extracted with solvents. It is employed for those plant materials for which no chemical or biological assay method exists. The extraction of any crude drug with a particular solvent yields a solution containing different phytoconstituents. The composition of these phytoconstituents depends on the nature of drug and solvent used. The use of a single solvent can be the means of providing preliminary information about the quality of a particular drug sample 8.
TABLE 1: EXTRACTIVE VALUES OF LEAVES OF CLITORIA TERNATEA IN DIFFERENT SOLVENTS
| Solvent Used | Extractive value (% w/w) |
| Distilled water | 12.46 |
| Petroleum ether (60-80) | 2.46 |
| Chloroform | 6.88 |
| Ethyl acetate | 9.84 |
| Methanol | 14.42 |
Preliminary Phytochemical Screening: Pre-liminary tests were carried out for the presence or absence of phytoconstituents like alkaloids, carbohydrates, flavonoids, glycosides, saponins, sterols, terpenes and tannins in all the extracts by using the above four solvents individually. A description of methods adopted for performing the tests are summarized below.
Test for Alkaloids: A portion of the extract was made acidic with dilute sulphuric acid. This portion was divided into two parts and was tested with the following precipitating reagents:
Mayer’s Reagent: 1.36 gm of mercuric chloride dissolved in 60 ml of water, was added in a solution of 5 gm of potassium iodide in 20 ml of distilled water. They are mixed properly and volume was made up to 100 ml with distilled water. The buff colored precipitate was considered to be a positive test 9.
Dragendorff Reagent: 1 gm of bismuth subnitrate in 20 ml of acetic acid was added to 20 grams of potassium iodide in 100 ml of water. The orange or brown colored precipitate was considered to be positive test 10.
Test for Carbohydrates:
Molisch’s Test: It was performed for the confirmation of carbohydrates. 1 ml of 10% of acidic solution of α-naphthol was added to the extracts & mixed. Then 1 ml of concentrated sulphuric acid was carefully poured along the sides of the test tubes. The violet ring at the juncture of the two layers was considered to be positive test 11.
Test for Glycosides:
Kedde Test (For Aglycone): The extract was evaporated to dryness and one drop of 90% alcohol and two drops of 2% 3, 5 dinitrobenzoic acids in 90% alcohol was added and the above mixtures was made alkaline with 20% Sodium hydroxide to get the purple color. The appearance of the purple color showed the presence of free aglycone moiety 12.
Keller–Killani Test (For Sugar): To the dried extract 0.4 ml of glacial acetic acid containing a trace of Ferric chloride was added. To the mixture, 0.5 ml of concentrated H2SO4 was added. The presence of green-blue color in the upper acetic acid layer indicates the presence of sugar moiety 13.
Test for Flavonoids: The extract was treated with magnesium (dust) and concentrated HCl. The appearance of a pink tomato color was indicative the presence of Flavonoids.
Shinoda’s Test: 5 - 10 drops of dilute hydrochloric acid were added to 0.5 ml of extract. A small piece of magnesium was added to it. The appearance of pink, reddish-pink or brown coloration was considered positive test. The appearance of yellow, orange-red or brick color precipitate with lad acetate indicates the presence of Flavonoids 14. 10 ml solution of the extract was hydrolyzed with dilute sulphuric acid. This was extracted with ether and divided into two portions. 1 ml dilute ammonia solution was added to one portion, a greenish-yellow color conform presence of Flavonoids. To the other portion 1 ml of dilute sodium bicarbonate solution was added; a pale yellow color conforms to the presence of Flavonoids.
Test for Sterols and Terpene:
Benedict’s test: To 5 ml of Benedict’s reagent few drops of the extract were added and boiled in water bath for 5 min. The appearance of a green, yellow or orange-red precipitate indicates the presence of reducing sugar 15.
Fehling’s Test for Reducing Sugars: To the equal volume of Fehling A and Fehling B mixture, 2 ml of extract was added and boiled for 5 min in a water bath. The appearance of red precipitate indicates the presence of reducing sugar.
Test for Saponins:
Foam Test: A small amount of dry extract was boiled with water and allowed to cool. It was then shaken vigorously for a minute. The formation of persistent honeycomb-like forth was taken as positive results for Saponins 16.
Test for Tannins: A small portion of the extract was treated with 5% ferric chloride solution appearance of green to blue color is a positive test for tannins. A creamy precipitate with lead acetate was considered positive test for tannins.
TABLE 2: RESULT OF PRELIMINARY PHYTOCHEMICAL SCREENING OF EXTRACTS FOR VARIOUS PHYTOCONSTITUENTS
| Extracts | Alkaloid | Carbohydrates | Flavonoid | Glycosides | Red sugar | Saponins | Sterols | Terpenes | Tannins |
| Petroleum ether | -- | -- | -- | ++ | -- | -- | ++ | ++ | -- |
| Choloroform | + | -- | -- | + | ++ | - | + | -- | + |
| Ethyl acetate | -- | + | +++ | + | -- | + | -- | -- | -- |
| Methanol | +++ | + | +++ | ++ | ++ | + | + | +++ | -- |
| Water | +++ | +++ | +++ | ++ | ++ | -- | -- | -- | ++ |
+++ Prominently present; ++ Moderately present, + Slightly present, -- Absent
Acute Toxicity & Gross Behavioural Studies: Acute toxicity studies were carried out for methanolic extract using the Acute Toxic Method as described in OECD (Organization of Economic Cooperation & Development) Guide Line No: 423. Animals were given increasing doses of 30, 100, 300, 600 & 1000 mg/kg p.o of the methanolic extract suspended in 2% tween-80 solution. The animals were observed continuously for 2 h gross behavioral changes and intermittently once every 2 h and finally at the end of 24 and 72 h to note any toxic sign 17.
Experimental Design:
Group I: Negative control (Normal Saline)
Group II: Positive control (CCl4 1 ml/kg/day) 9 days
Group III: Standard (Silymarin 100 mg/kg/day + CCl4 1 ml/kg/day) 9 days
Group IV: Test (1) (Methanolic extract of Clitoria ternatea 500 mg/kg/day + CCl4 1 ml/kg/day) 9 days.
Group V: Test (2) (Methanolic extract of Clitoria ternatea 1000 mg/kg/day + CCl4 1 ml/kg/day) 9 days.
Assessment of Liver Function: The Hepatoprotective effect of the extract was evaluated by the assay of Liver function biochemical parameters namely Serum glutamate pyruvate transaminase (SGPT), Serum glutamate oxaloacetate transaminase (SGOT), alkaline phosphates (ALP) & Total serum bilirubin (SB) according to the standard methods.
Histopathological Studies: Formalin-fixed tissues were subjected to graded dehydration in ascending strength of alcohol 70%, 80%, 90% and 100% and a subsequent wash with xylene. Following these the tissues were embedded in liquid paraffin to facilitate preparation of histopathological blocks which are essential for imparting strength to the tissue so that it can withstand the abrasive force of the blade while being sectioned. The 5 µm sections which were obtained by trimming with manual rotary microtome are subjected to staining by Harris haematoxylin and counterstained by Eosin. The sections were viewed under trinocular microscope of different magnifications which were photographed by Motif software inbuilt in systems 22.
Statistical Analysis: Results have been expressed as mean ± SEM. One way ANOVA has been employed for comparing majority of parameters. Post hoc tests were used for identification of groups having significant differences for one way ANOVA. Turkey’s Multiple Range Test was used for comparisons. Whereas for two way ANOVA Bonferroni’s test was used for the post hoc analysis. The significant groups were identified on the figure by designated alphabets 23.
Nephroprotective Activity: Nephrotoxicity can be defined as renal disease or dysfunction that arises as a direct or indirect result of exposure to medicines and industrial or environmental chemicals. Drug nephrotoxicity is, therefore, any renal dysfunction attributable to drugs.
Drug nephropathies are not restricted to a single type of renal injury. Drugs target one or more discrete anatomical regions of the kidney and may affect only one cell type. The resulting insult to the kidney may result in a spectrum of nephropathies that are indistinguishable from those that do not have chemical etiology. The nephron is the functional unit of the kidney and consists of a continuous tube of highly specialized heterogeneous cells, which show sub-specialization along the length of nephron and between them.
It is the major organ of excretion and homeostasis for water-soluble molecules because it is a metabolically active organ; it can concentrate certain substances actively. Also, its cells have the potential to bio convert chemicals and metabolically activate a variety of compounds. Since the kidney excretes many drugs, it is routinely exposed to high concentrations of these drugs or their metabolites or both 24.
Furthermore, the kidney has several features that allow nephrotoxins to accumulate. It is highly vascular, receiving about 25% of the resting cardiac output. The proximal renal tubule presents a large area for nephrotoxin binding and transport into the renal epithelium. Reabsorption of the glomerular filtrate progressively increases intraluminal nephrotoxin concentrations, while specific transport pathways in the kidney may engender site-specific toxicity. Free radicals are highly reactive substances formed in the body as a result of metabolic processes 25. Many of these molecular species are oxygen (and sometimes nitrogen) centered free radical and its non radical products. The term reactive oxygen species (ROS) collectively denotes oxygen centered radicals (superoxide and hydroxyl radicals) as well as non radical species derived from oxygen such as hydrogen 1 peroxide, singlet oxygen (O).
The increased production of ROS seems to accompany most forms of tissue injury. Free radical can also react with DNA, proteins or lipids in the cell membrane and cause damage. The involvement of ROS in aging and in many chronic diseases has been considered. The defense provided by anti-oxidant system is crucial for the survival of organisms. Detoxification of ROS in the cell is provided by both enzymatic and non-enzymatic systems, which constituent the antioxidant defense system.
Many plants contain antioxidant compounds, and these compounds protect cells against the damaging effects of reactive oxygen species (ROS) such as singlet oxygen, superoxide, peroxyl radicals, hydroxyl radicals, and peroxynitrite. These compounds or anti-oxidant that can scavenge free radicals have vital role in improvement of diseased conditions 26, 27.
Experimental Design:
Group 1: Negative control (Normal Saline).
Group 2: Positive control Cisplatin (16 mg/kg b.w single dose i.p).
Group 3: Standard Cystone (5 ml/kg) + Cisplatin (16 mg/kg b.w single dose i.p).
Group 4: Test (1) Methanolic extract of Clitoria ternate (500 mg/kg/day, po ) + Cisplatin (16 mg/kg b.w single dose i.p).
Group 5: Test (2) Methanolic extract of Clitoria ternatea (1000 mg/kg /day, po) + Cisplatin (16 mg/kg b.w single dose i.p).
Assessment of Renal Function: The nephroprotective effect of extract was evaluated by the assay of kidney function biochemical para-meters such as kidney weight as % of the total body weight, Blood urea level, serum creatinine level and Histopathological evaluation of the kidney 28.
Statistical Analysis: Results have been expressed as mean ± SEM. One way ANOVA has been employed for comparing majority of parameters. Post hoc tests were used for identification of groups having significant differences for one way ANOVA. Turkey’s Multiple Range Test was used for comparisons. Whereas for two-way ANOVA Bonferroni's test was used for the post hoc analysis. The significant groups were identified on the figure by designated alphabets 29, 30, 31.
TABLE 3: SERUM BIOCHEMICAL ANALYSIS RESULT OF HEPATOPROTECTIVE ACTIVITY
| Group | Treatment region | SGPT(IU/L) | SGOT(IU/L) | ALP(IU/L) | Serum bilirubin(IU/L) |
| 1 | Normal saline | 62.58 ± 4.12 | 155.45 ± 6.32 | 264.35 ± 8.22 | 0.42 ± 0.04 |
| 2 | CCl4 | 240.58 ± 13.54a | 490.68 ± 15.58a | 534.82 ± 19.43a | 1.42 ± 0.32a |
| 3 | Silymarin + CCl4 | 73.42 ± 5.45*** | 172.43 ± 6.75*** | 283.76 ± 10.41*** | 0.45 ± 0.04*** |
| 4 | MCT 500 mg/kg + CCl4 | 115.45 ± 7.56*** | 226.43 ± 8.68*** | 324.96 ± 12.47*** | 0.62 ± 0.05*** |
| 5 | MCT 1000 mg/kg + CCl4 | 92.65 ± 7.42*** | 205.54 ± 8.62*** | 301.82 ± 10.32*** | 0.52 ± 0.05*** |
Values are expressed as mean ± SEM (n=6) and analyzed by using ANOVA followed by Bonferroni's multiple comparison test. Percentage change of protective effect compared with the CCl4 treated control is expressed within brackets. P: a< 0.001 vs. vehicle control, ns> 0.05, < 0.05, <0.01, < 0.001 vs. CCl4 treated control.
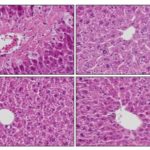
FIG. 1: HISTOPATHOLOGICAL EVALUATION OF HEPATOPROTECTIVE ACTIVITY
Group1: Negative control (Normal saline).
Group 2: Positive control (CCl4).
Group 3: Standard (Silymarin + CCl4).
Group 4: Test (1) (Methanolic extract of Clitoria ternatea 500 mg/kg + CCl4).
Group 5: Test (2) (Methanolic extract of Clitoria ternatea 1000 mg/kg + CCl4).
TABLE 4: SERUM BIOCHEMICAL ANALYSIS RESULT FOR NEPHROPROTECTIVE ACTIVITY
| Group | Treatment Regimen | Urea (mg/dl) | Creatinine (mg/dl) |
| 1 | Normal Saline | 38.76 ± 3.76 | 1.06 ± 0.40 |
| 2 | Cisplatin | 75.7 1 ± 7.50a | 2.51 ± 0.88a |
| 3 | Cystone 5 ml/kg + Cisplatin 16 mg/kg b.w | 52.57 ± 4.56*** | 1.36 ± 0.62* |
| 4 | Clitoria ternatea 500 mg/kg + Cisplatin 16 mg/kg i.p | 68.74 ± 5.46*** | 1.82 ± 0.83ns |
| 5 | Clitoria ternatea 1000 mg/kg/day + Cisplatin 16 mg/kg b.w .single dose i.p | 57.43 ± 7.34*** | 1.55 ± 0.42* |
Values are expressed as mean ± SEM (n = 6) and analyzed by using ANOVA followed by Bonferroni's multiple comparison test. Percentage change of protective effect compared with the Cisplatin treated control is expressed within brackets. p: a< 0.001 vs. vehicle control, NS> 0.05, *< 0.05 , ** <0.01 ,***< 0.001 vs. Cisplatin treated control.
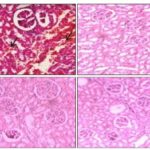
FIG. 2: HISTOPATHOLOGICAL EVALUATION OF NEPHROPROTECTIVE ACTIVITY
Group 1: Negative control (Normal saline).
Group 2: Positive control (Cisplatin 5 ml).
Group 3: Standard (Cystone5 ml + Cisplatin 16 mg/kg).
Group 4: Test (1) (Methanolic extract of Clitoria ternatea 500 mg/kg + Cisplatin16 mg/kg).
Group 5: Test (2) (Methanolic extract of Clitoria ternatea 1000 mg/kg + Cisplatin 16 mg/kg).
DISCUSSION: In the present study it was found that the methanolic extract Clitoria ternatea can modulate the nephrotoxicity induced by CCl4.
The hepatotoxicity of CCl4 has been reported to be due to the formation of the highly reactive trichloro (CCl3) free radical, which alters function of endoplasmic reticulum and causes peroxidative degradation of lipid membrane of the adipose tissue leads to loss of metabolic enzyme located in the intracellular structures. Further it has been evident that several phytoconstituents can induce microsomal enzymes either by accelerating the excretion of CCl4 or by inhibition of lipid peroxidation induced by CCl4. Phytoconstituent menstruum namely flavonoids, triterpenoids, saponins and alkaloids are known to possess hepatoprotective activity.
In this study, the CCl4 induced Liver damage was characterized by an increased level of SGPT, SGOT, ALT, and SB. Pre-treatment of animal with silymarin 100 mg/kg p.o. could reduce the level of SGPT, SGOT, ALP and SB. Similar results were obtained by pre-treatment of animal with methanolic extract of Clitoria ternatea (500 mg/kg & 1000 mg/kg p.o). Compared with the CCl4 treated group. However the effectiveness of the extract the dose levels tested was less compared to the standard Hepatoprotective drug used in the study, silymarin.
Silymarin has very low toxicity and has been shown to possess a good safety profile. At high doses, a laxative effect is observed due to increased bile secretion and bile flow. Adverse of patients in a clinical trial. Serious adverse effects, which are rare, include gastroenteritis associated with collapse and allergy. Thus combination therapy may up to some extent reduce these adverse effects of silymarin.
It is evident from the studies carried out by earlier researchers that the tuber of the plant contained higher phenolic and Flavonoids content and scavenging activities. The qualitative phyto-chemical investigations carried out on the methanolic extract of Clitoria ternatea have significant hepatoprotective activity. This may be probably due to the higher content of flavonoids.
In the present study, it was found that the tuber extract of Clitoria ternatea can modulate the nephrotoxicity induced by cisplatin. Cisplatin is a potent drug used in the management of wide range of cancer. However, the severe toxic side effects are the major limitation in its usage although the mechanism of cisplatin-induced nephrotoxicity is exactly unknown. Several studies have suggested that the nephrotoxicity is induced by lipid peroxidation and free radicals. On the other hand many antioxidants have been shown to be protective against cisplatin-induced nephrotoxicity. Also various free radicals scavengers have been shown to be effective in protection against cisplatin-induced nephrotoxicity & treatment with such agents provide significant protection against cisplatin-induced acute renal failure. In this study the cisplatin-induced kidney damage was characterized by significant increase in serum creatinine (p<0.01) and urea (p<0.001) in comparison with the control group. Pre-treatment of animal with cystone (5 ml/kg p.o) for 6 consecutive days & a single dose of cisplatin (16 mg/kg i.p) prevented the elevation of urea & serum creatinine as compared with cisplatin-induced nephrotoxicity group.
However, pre-treatment of animal with methanolic extract of Clitoria ternatea (500 mg/kg) for 6 consecutive days and a single dose of cisplatin did not cause a significant reduction of serum creatinine as compared with cisplatin-induced nephrotoxicity group. The histopathological evaluation of the kidney preparations in treatment group also revealed a decreased induced tubular congestion, tubular cast, epithelial desquamation, glomerular congestion, blood vessel congestion & inflammatory cells.
CONCLUSION: The methanolic extract of leaves of Clitoria ternatea 1000 mg/kg was found to possess hepatoprotective and nephroprotective activity. The activity was found to be less compared to the standard drugs used in the study. The studies were done using the crude lyophilized extract.
ACKNOWLEDGEMENT: Avik Das, Tathagata Roy, Preeta Bose they have a plethora of contribution to this scientific research.
CONFLICT OF INTEREST: Nil
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
REFERENCES:
- Brown AC, Reitzenstein JE, Liu J and Jadus MR: Hepatoprotective activity of Colocasia esculenta in mice. International Journal of Pharmaceutical Science & Drug Research 2005; 19: 767-71.
- Kale BP, Kothekar MA, Tayade HP, Jaju JB and Mateenuddin M: Effect of aqueous extract of Azadirchta indica leaves on Hepatotoxicity induced by anti-tubercular drugs in rats. Indian Journal of Pharmacology 2003; 35: 177-80.
- Marcinek K and Krejpcio Z: Stevia rebaundiana bertonia chemical composition & functional properties. Acta Sci Pol Aliment 2015; 14(2): 145-52.
- Devmurari VP and Jivani NP: Hepatoprotective activity of methanolic & aqueous extract of Azadirchata indica International Journal of Pharm Tech Research 2010; 2(2): 1037-40.
- Ruiz–Ruiz JC, Moguel-Ordonez YB and Segura-Campos MR: Biological activity of Stevia rebaundiana Bertoni & their relationship to health. Critical Reviewers in Food Science & Nutrition 2017; 57(12): 2680-90.
- Robin S, Sunil, Rana AC and Nidhi S: Different models of hepatotoxicity and related liver diseases: a review. Int Research Journal of Pharmacy 2012; 3(7): 86-95.
- Wolwer-Rieck U: The leaves of Stevia rebaundiana, their constituents & the analyses there of: a review. Journal of Agricultural & Food Chemistry 2012; 60(4): 886-95.
- Yadav PN, Pal A, Shanker K, Bawankule UD, Gupta KA, Darokar PM and Khanuja SPS: Synergistic effect of silymarin and standardized extract of Phyllanthus amarus against CCl4 induced heptotoxicity of rattus norvegicus. Phytomedicine 2008; 15: 1053-61.
- Goswami S and Singhai R: Activity of Moringa oleifera using successive solvent extraction technique. Int Journal of Theoretical & Applied Sciences 2016; 8(1): 23-27.
- Govindappa PK, Gautam V, Tripathi SM, Sahni YP and Raghavendra HLS: Effect of Withania somnifera on gentamicin induced renal lesions in rats. Revista Brasileira de Farmacognosia 2019; 29(2).
- Pal S, Bhattacharjee A, Mukherjee, Bhattacharya K and Khowala S: Anti-oxidant and Hepatoprotective activity of ethanolic extract of Alocasia indica AJPCT 2014; 2(2): 191-08
- Abraham Z, Bhakuni DS, Garg HS, Goel AK, Mehrota BN and Patnaik GK: Screening of Indian plants for biological activity. Exp Biol Indian J 986; 24(1): 48-68.
- Chattapadhyay RR, Sarkar SK, Ganguly S, Banerjee RN, Basu TK and Mukherjee A: Hepatoprotective activity of Azadirchta indica leaves on paracetamol-induced hepatic damage in rats. Ind J of Exper Bio 1992; 30(8): 738-40.
- Harborne JB, Chapman and Hall: Phytochemical Methods. London 1983; 2: 277-9.
- Iwashina T, Konishi T, Takayama A, Fukada M and Ootani S: Isolation and identification flavonoids in the leaves of taro. Ann Tsu Botanical Garden 1999; 18: 71-4.
- Hung AS and Tanudjaja LS: Application of anion exchange high performance liquid chromatography in determining oxalates in taro (Colocasia esculenta) corms. J Agric Food Chem 1992; 40: 2123-6.
- Zhang Q, Yang H, Li Y, Liu H and Jia X: Toxicological evaluation of ethanolic extract from Stevia rebaundiana Bertonia leaves: genotoxicity & subchronic oral toxicity. Regulatory Toxic & Pharmacology 2017; 86: 253-59.
- Baligar NS, Aladakatti RH, Ahmed M and Hiremath MB: Hepatoprotective activity of the neem-based constituent azadirchtin - a in carbon tetrachloride intoxicated Wistar rats. Canad J of Physio & Pharmacology 2014; 92: 267-77.
- Jahan IA, Ahmed KS, Sultana Z, Hossain MH, Biswas PK and Nada K: Anti-oxidant activity of methanolic extract of Withania sominifera in Mice. Oriental Pharmacy & Experimental Medicine 2018; 18: 299-07.
- Falowo AB, Muchenje V, Hugo A, Aiyegoro OA and Fayemi PO: Anti-oxidant activities of Moringa oleifera & Bidens pilosa leaf extracts & their effects on oxidative stability of ground raw beef during refrigeration storage CYTA. Journal of Food 2016; 15(2): 249-256.
- Shah BN, Nayak BS, Bhatt SP, Jalalpure SS and Sheth AK: The anti-oxidant activity of leaves of Colocasia esculenta. Pharmacognosy Journal 2007; 15: 3-4.
- Pakade V, Cukrowska E and Chimuka L: Comparison of antioxidant activity of Moringa oleifera & selected vegetables in South Africa. South African Journal of Science 2013; 109: 95-99.
- Jeyanthi T and Subramanian P: Nephroprotective effect of Withania somnifera: a dose-dependent study. Renal Failure 2009; 31(9): 814-21.
- Padhya MR, Jogdand SD and Bhattacharjee J: Evaluation & comparison of nephroprotective effect of Hemidesmus indicus & Withania somnifera Linn. on gentamicin induced nephrotoxicity in rats. International Journal of Basic & Clinical Pharmacology 2018; 7: 691-95.
- Kaushwaha V, Sharma M, Vishwakarma P, Saini M and Saxena K: Biochemical assessment of nephroprotective & nephrocurative activity of Withania somnifera on gentamicin-induced nephrotoxicity in experimental rats. International Journal of Research in Medical Sciences 2016; 4(1): 298-02.
- Shimeda Y, Hirotanic Y, Akimoto Y, Shindou K, Yoshio I, Nishihori T and Tanaka K: Protective effects of capsaicin against cisplatin-induced nephrotoxicity in Rats. Biol Pharm Sci 2005; 28(9): 1635-8.
- Preethi CK and Kuttan R: Hepato and Reno Protective action of Calendula officinalis flower extract. Ind J Exp Biol 2009; 47: 163-8.
- Kalariya M, Parmar S and Sheth N: Neuropharmacological activity of hydroalcoholic extract of leaves of Stevia rebaundiana. Biol Pharm 2010; 48: 1207-12.
- Ghori SS, Siddiqua TS and Anees Fathima: Nephroprotective effect of Ficus Dalhousiae Miq leaf methanolic extract in Albino Wistar Rats. International Journal of Pharma Research & Health Sciences 2016.
- Kamkaen N and Wilkinson JM: The anti-oxidant activity of Clitoria ternatea flower petal extracts & eye gel. Wiley Online Library 23rd Oct 2009; 23.
- Chong FC and Gwee XF: Ultrasonic extraction of anthocyanin from Clitoria ternatea flowers using response surface methodology. Nat Prod Res 2015; 29(15): 1485-7.
How to cite this article:
Srikanta Chandra, Avik Das, Tathagata Roy, Preeta Bose, Lucky Mukherjee and Jyotirmay Samanta: Evaluation of methanolic extract of Clitoria ternatea hepato-protective and nephroprotective activity in rats. Int J Pharmacognosy 2019; 6(9): 310-18. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.6(9).310-18.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
310-318
780
1279
English
IJP
S. Chandra *, A. Das, T. Roy, P. Bose, L. Mukherjee and J. Samanta
Department of Pharmacology, Jakir Hossain Institute of Pharmacy, Raghunathganj, Murshidabad, West Bengal, India.
Srikanta.chandra95@gmail.com
25 August 2019
23 September 2019
27 September 2019
DOI: 10.13040/IJPSR.0975-8232.IJP.6(9).310-18
30 September 2019