EVALUATION OF ANTIDIABETIC ACTIVITY OF HETEROPHRAGMA QUADRILOCULARE (ROXB.) K. SCHUM. LEAVES
HTML Full TextEVALUATION OF ANTIDIABETIC ACTIVITY OF HETEROPHRAGMA QUADRILOCULARE (ROXB.) K. SCHUM. LEAVES
B. H. Satani * 1, V. S. Surana 1, S. A. Shah 1 and S. H. Mishra 2
Maliba Pharmacy College 1, Uka Tarsadia University, Bardoli-Mahuva Road, Bardoli - 394350, Gujarat, India.
Faculty of Pharmacy 2, The M. S. University of Baroda, Vadodara - 390002, Gujarat, India.
ABSTRACT: Heterophragma quadriloculare (Roxb.) K. Schum. (HQ) belongs to Bignoneace family and known as Warras. Usage of HQ plant in diabetes has been claimed traditionally, but scientific documentation is not yet available. So, it was our interest to develop a scientific database for HQ leaves. HQ leaves were studied for its traditional claim of anti-diabetic activity by oral glucose tolerance test (OGTT) model using normal rats. All possible phytoconstituents were extracted into a selected range of solvents based on its polarity, i.e., petroleum ether, chloroform, methanol, water. The extracts were screened for OGTT at a dose level of 200 and 400 mg/kg per oral. In OGTT study through all extracts found to reduce blood glucose level, petroleum ether extract and chloroform extract showed dose-dependent activity. These results provided the clue for the presence of anti-diabetic constituents in petroleum ether extract. Thus petroleum ether extract (200 mg/kg), an unsaponifiable fraction of petroleum ether extract (100 mg/kg) and a saponifiable fraction of petroleum ether extract (100 mg/kg) of HQ were studied in rats by STZ-NAD induced type-II diabetic model up to 21 to find out the most potent anti-diabetic fraction. Blood glucose and lipid profile were recorded on 0th and 21st day. Oral administration of PEHQ and UPEHQ resulted in significant weight gain, reduction of blood glucose, serum cholesterol, serum VLDL and triglycerides in diabetic rats as compared to diabetic control rats. The unsaponifiable fraction of petroleum ether extract at the dose of 100 mg/kg showed significant antidiabetic activity. The present study thus justifies the traditional claim of Heterophragma quadriloculare (Roxb.) K. Schum. for diabetes. The unsaponifiable fraction of petroleum ether extract of HQ needs further attention for separation and identification of biologically active compounds that are unidentified yet.
| Keywords: |
Heterophragma quadriloculare (Roxb.) K. Schum, Anti-diabetic, Oral glucose tolerance test, Streptozotocin nicotinamide induced Type 2 diabetes
INTRODUCTION: H. quadriloculare (Roxb.) K. Schum. (HQ) belongs to Bignoneace family and known as Warras 1. In India it is found in different regions of Madhya Pradesh, Maharashtra, Tamil Nadu, Karnataka, Gujarat and Andhra Pradesh 2 - 4.
This plant is utilized as anti-diabetic, antifungal, antiseptic and in skin disease like toe sores and chilblain 5. The utility of HQ plant material has been claimed traditionally by many authors, but scientific documentation is not yet available.
So, it was our thought of interest to develop a scientific database for HQ leaves. With this objective anti-diabetic activity of HQ, leaves have been evaluated by in-vivo screening of different extracts of leaves for oral glucose tolerance test in normal rats followed by in-vivo screening of different extracts of leaves for anti-diabetic activity in streptozotocin (STZ) - nicotinamide (NAD) induced type-II diabetic rat.
MATERIALS AND METHODS: Petroleum ether extract (PEHQ), chloroform extract (CHQ), aqueous extract (AqHQ) and methanolic extract (MeHQ) obtained by successive solvent extraction of HQ leaves have been screened for oral glucose tolerance test (OGTT) and petroleum ether extract (PEHQ), unsaponifiable fraction of petroleum ether extract (UPEHQ) and saponifiable fraction of petroleum ether extract (SPEHQ) of HQ leaves have been screened by streptozotocin-nicotinamide induced type-II diabetic rat model. Whenever required, 0.5% tween 80 in normal saline solution was used as a vehicle.
Chemicals: Streptozotocin, nicotinamide, and metformin hydrochloride were obtained from HiMedia laboratories (Mumbai, India). Kit for biochemical estimations was procured from Span Diagnostics Ltd., India and Reckon Diagnostics Pvt. Ltd., India. Other experimental chemicals used were of analytical grade and purchased from Loba Chemie Pvt. Ltd. (Mumbai, India).
Plant Material Collection, Processing and Preparation of Extracts: The fresh leaves of wildly growing HQ were collected from the outskirts of village Mota Randha, Dadara and Nagar Haveli, during April-May 2010 and authenticated Dr. J.K. Vanparia, Botanist, VNSGU, Surat. A voucher specimen of the sample (No. Pharmacy / HDT/HQ/09-10/01/BS) has been deposited in Herbal Drug Technology Laboratory, Pharmacy Department, The M. S. University of Baroda, Gujarat, India for database.
Collected leaves were shed dried for two week, powdered in mechanical grinder and stored in air tight container. HQ leaves (2000 g) was extracted with petroleum ether followed by chloroform and methanol using Soxhlet apparatus. Remaining plant material was extracted with water by decoction. Each extract was dried (45 °C) in a rotary evaporator (Heidolph, Germany). The dried extracts were stored in a vacuum desiccator until further use. PEHQ (20 g) was saponified by refluxing it with 10% alcoholic KOH for 2 h. After saponification saponifiable fraction (SPEHQ) was separated and unsaponifiable matter (UPEHQ) was extracted in diethyl ether (3 × 100 ml). The unsaponifiable matter was collected as a solid orange mass with sticky nature.
Experimental Animals: Healthy female Wistar albino rats weighing 200 - 250 gm, procured from Zydus Cadilla Research Laboratory, Ahmedabad maintained under standard husbandry conditions (Temperature 23 ± 2 °C, relative humidity 55 ± 10% and 12 h light-dark cycle). The animals were fed with standard rat pellet diet and had free access to water. The experimental protocols were approved by the Institutional Animal Ethics Committee, The M. S. University of Baroda, Vadodara, Gujarat (CPCSEA Reg. No. 404/PO/Re /S/01/CPCSEA). All studies were conducted as per the National Institute of Health’s Guide for the Care and Use of Laboratory Animal.
In-vivo Screening for Oral Glucose Tolerance Test in Normal Rats:
Experimental Design: The oral glucose tolerance test for selected extracts was performed on overnight (18 h) fasted normal rats. In this experiment, 60 normal rats were used. They were separated into ten groups of 6 rats each. Extracts were dissolved in the vehicle.
Group I (P1): Rats were given PEHQ (200 mg/kg b.w.) orally.
Group II (P2): Rats were given PEHQ (400 mg/kg b.w.) orally.
Group III (C1): Rats were given CHQ (200 mg/kg b.w.) orally.
Group IV (C2): Rats were given CHQ (400 mg/kg b.w.) orally.
Group V (M1): Rats were given MeHQ (200 mg/kg b.w.) orally.
Group VI (M2): Rats were given MeHQ (400 mg/kg b.w.) orally.
Group VII (A1): Rats were given AqHQ (200 mg/kg b.w.) orally.
Group VIII (A2): Rats were given AqHQ (400 mg/kg b.w.) orally.
Group IX (control): Rats were given Vehicle (quantity sufficient) orally.
Group X (metformin): Rats were given metformin HCl (15 mg/kg b.w.) orally.
Glucose (3 g/kg) was fed orally in solution form to each group 30 min after the administration of extracts and metformin HCl 6.
Biochemical Analysis: Blood was withdrawn using hematocrit capillary from the retro-orbital plexus under ether inhalation anaesthesia at - 30, 0, 30, 60, and 120 min of glucose administration. Collected blood was allowed to coagulate and centrifuged at 5,000 RPM for 15 min. The glucose level in serum was estimated by glucose oxidase-peroxidase method using a reagent kit.
Statistical Analysis: All results were reported as mean ± SEM. The variation in a set of data has been estimated by performing Bonferroni repeated measures one-way ANOVA using non-parametric methods in Graph pad prism.
In-vivo Screening for Anti-Diabetic Activity on STZ - NAD Induced Type-II Diabetic Rat:
Experimental Induction of Diabetes in Rats: Diabetes was induced by a single intraperitoneal injection of 230 mg/kg nicotinamide prepared in normal saline followed by freshly prepared streptozotocin (65 mg/kg) in 0.1 M citrated buffer (pH = 4.5) to overnight starved rats. Diabetic rats were provided with 20% glucose solution to drink overnight to prevent the initial drug-induced hypoglycaemic death. Blood glucose was estimated after 7 days and animals with glucose level >180 ± 8 mg/dl were only selected for the study 6 - 9.
Experimental Design: In this experiment, 30 diabetic rats were used. They were separated into five groups of 6 rats each. Extracts and fractions were dissolved in the vehicle for administration.
Group I (Diabetic Control): Rats were given Vehicle (quantity sufficient) orally for 21 days.
Group II (STD): Rats were given metformin HCl (15 mg/kg b.w.) orally for 21 days.
Group III (PEHQ): Rats were given PEHQ (200 mg/kg b.w.) orally for 21 days.
Group IV (UPEHQ): Rats were given UPEHQ (100 mg/kg b.w.) orally for 21 days.
Group V (SPEHQ): Rats were given SPEHQ (100 mg/kg b.w.) orally for 21 days.
Blood was withdrawn using hematocrit capillary from the retro-orbital plexus under ether inhalation anaesthesia. Fasting blood glucose, total cholesterol, VLDL, triglyceride, and body weight were recorded on 0th and 21st day.
Biochemical Analysis: Glucose level in serum was determined by glucose oxidase/peroxidase method using reagent kit from Span Diagnostic 10. Total cholesterol, serum VLDL and triglyceride were determined by standard methods using a regent kit from Reckon Diagnostics 11.
Statistical Analysis: All results were reported as mean ± SEM. The variation in a set of data has been estimated by performing Bonferroni repeated measures one-way ANOVA using non-parametric methods in Graph pad prism.
RESULTS AND DISCUSSION:
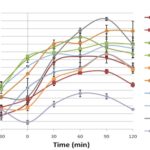
In-vivo Screening for Oral Glucose Tolerance Test in Normal Rats: Antidiabetic activity of the aerial part of the plant H. quadriloculare (Roxb.) K. Schum. (HQ) is traditionally reported by Soumyanath Amla 12. While the phytochemical study showed the presence of carbohydrates in leaves. So there was a question that despite the presence of carbohydrates in leaves how aerial part could be used as an anti-diabetic agent? But this ambiguity was little cleared by results of OGTT study Fig. 1.
FIG. 1: BLOOD GLUCOSE LEVEL OBTAINED IN OGTT STUDY. P1- PEHQ (200 mg/kg), P2- PEHQ (400 mg/kg), C1- CHQ (200 mg/kg), C2- CHQ (400 mg/kg), M1- MEHQ (200 mg/kg), M2- MEHQ (400 mg/kg), A1- AQHQ (200 mg/kg), A2- AQHQ (400 mg/kg)
Though all extracts found to reduce blood glucose level, PEHQ and CHQ showed dose-dependent activity. We have found that MeHQ and AqHQ did not reduce blood glucose level significantly as reduced by PEHQ and CHQ; this might be due to the presence of the high amount of carbohydrates in leaves and carbohydrates come out with aqueous and alcoholic extracts. This experiment showed probability of the presence of some anti-diabetic constituents in PEHQ thus PEHQ, UPEHQ and SPEHQ have been selected for chronic study to find the most potent anti-diabetic fraction.
In-vivo Screening for Anti-Diabetic Activity on STZ - NAD Induced Type-II Diabetic Rat: Chronic anti-diabetic study was carried out using STZ-NAD induced diabetic rats. Duration of study was 21 days. Change in body weight, blood glucose, serum cholesterol, serum VLDL and serum triglyceride observed at 0 and 21st day of study is explained in this section.
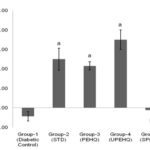
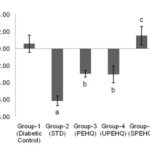
FIG. 2: CHANGE IN BODY WEIGHT OF RATS OBSERVED AT 0th AND 21st DAY OF TREATMENT. AP<0.0001 AS COMPARED TO DIABETIC CONTROL RAT. BP>0.05 AS COMPARED TO DIABETIC CONTROL RATS. STD - Metformin HCl (15 mg/kg), PEHQ- PEHQ (200 mg/kg), UPEHQ- UPEHQ (100 mg/kg), SPEHQ- SPEHQ (100 mg/kg)
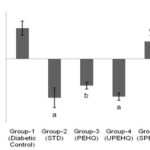
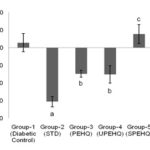
Loss in body weight was observed in STZ induced diabetic rats. STZ partly destroys the beta cells which decrease insulin secretion and produce type-II diabetes 13. Reduced body weight in diabetic rats affirms a corruption of basic proteins because of diabetes 14. The deficit in body weight noticed in STZ induced diabetic control rats may be due to muscle squandering 15-16. Oral administration of PEHQ and UPEHQ resulted in significant weight gain in diabetic rats while SPEHQ prevented further loss in body weight of diabetic rats as compared to diabetic control Fig. 2. Improvement in body weight by PEHQ and UPEHQ was comparable to the standard drug metformin HCl. In diabetes glucose metabolism is impaired and resulted in increased blood glucose level. Similarly increased blood glucose was observed in STZ induced diabetic rats. Oral administration of PEHQ and UPEHQ significantly reduced blood glucose as compared to diabetic control and was observed to comparable with standard drug metformin HCl. SPEHQ was not able to reduce blood glucose suggest that anti-diabetic phytochemicals are not present in this fraction Fig. 3.
FIG. 3: CHANGE IN BLOOD GLUCOSE LEVEL OF RATS OBSERVED AT 0th AND 21st DAY OF TREATMENT. AP<0.0001 AS COMPARED TO DIABETIC CONTROL RAT. BP<0.05 AS COMPARED TO DIABETIC CONTROL RATS. CP>0.05 AS COMPARED TO DIABETIC CONTROL RATS. STD- Metformin HCl (15 mg/kg), PEHQ- PEHQ (200 mg/kg), UPEHQ - UPEHQ (100 mg/kg), SPEHQ- SPEHQ (100 mg/kg)
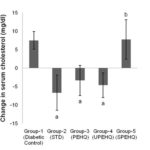
FIG. 4: CHANGE IN SERUM-CHOLESTEROL LEVEL OF RATS OBSERVED AT 0th AND 21st DAY OF TREATMENT. AP<0.05 AS COMPARED TO DIABETIC CONTROL RAT. BP>0.05 AS COMPARED TO DIABETIC CONTROL RATS. STD- Metformin HCl (15 mg/kg), PEHQ- PEHQ (200 mg/kg), UPEHQ- UPEHQ (100 mg/kg), SPEHQ- SPEHQ (100 mg/kg)
It is hypothesized that antidiabetic activity may be due to the presence of lupeol, ursolic acid, β-sitosterol and some other unidentified compounds 17, 18, 19, 20. The diabetic rats had found to have significantly higher chylomicron, VLDL, cholesterol, and triglyceride 21. In diabetes mellitus, metabolic derangements resulting from the absolute lack of insulin or resistance to the actions of insulin can affect VLDL and triglyceride metabolism.
FIG. 5: CHANGE IN SERUM VLDL LEVEL OF RATS OBSERVED AT 0th AND 21st DAY OF TREATMENT. AP<0.0001 AS COMPARED TO DIABETIC CONTROL RAT. BP<0.05 AS COMPARED TO DIABETIC CONTROL RATS. CP>0.10 AS COMPARED TO DIABETIC CONTROL RATS. STD - Metformin HCl (15 mg/kg), PEHQ- PEHQ (200 mg/kg), UPEHQ- UPEHQ (100 mg/kg), SPEHQ- SPEHQ (100 mg/kg)
FIG. 6: CHANGE IN SERUM TRIGLYCERIDE LEVEL OF RATS OBSERVED AT 0th AND 21st DAY OF TREATMENT. AP<0.0001 AS COMPARED TO DIABETIC CONTROL RAT. BP<0.05 AS COMPARED TO DIABETIC CONTROL RATS. CP>0.10 AS COMPARED TO DIABETIC CONTROL RATS. STD- Metformin HCl (15 mg/kg), PEHQ- PEHQ (200 mg/kg), UPEHQ- UPEHQ (100 mg/kg), SPEHQ- SPEHQ (100 mg/kg)
It has been reported that the outcome of this interplay between diabetes and VLDL metabolism is the common occurrence of elevated plasma VLDL and triglyceride concentrations in individuals with both Type 1 and Type 2 diabetes mellitus 22. In this study, serum cholesterol level was reduced by PEHQ and UPEHQ whereas SPEHQ was unable even to hinder increment of serum cholesterol level which was raised due to diabetic condition Fig. 4. Similarly, reduction in VLDL and serum triglyceride level was observed by PEHQ and UPEHQ as compared to diabetic control. SPEHQ administration leads to increment in VLDL level as well serum triglyceride level Fig. 5 and 6.
CONCLUSION: The result of OGTT study showed highly active fraction was petroleum ether extract followed by chloroform. The activity of both these extract was observed to be in a dose-dependent manner. While activity shown by methanol and water extract was inversely proportional to the dose. It was due to the presence of carbohydrates in plants. The petroleum ether extract (PEHQ), unsaponifiable fraction of petroleum ether extract (UPEHQ) and a saponifiable fraction of petroleum ether extract (SPEHQ) of HQ were studied in-vivo for anti-diabetic activity in rats by STZ-NAD induced type-II diabetic model up to 21 days. Results of the study demonstrated that unsaponifiable fraction of petroleum ether extract (UPEHQ) at the dose of 100 mg/kg possesses anti-diabetic activity.
The present study thus justifies the traditional claim of Heterophragma quadriloculare (Roxb.) K. Schum. for the treatment of diabetes and points out that it requires future detail investigation. The unsaponifiable fraction of petroleum ether extract of HQ also needs further attention for separation and identification of biologically active compounds that are unidentified yet.
ACKNOWLEDGEMENT: Authors are thankful to Zydus Cadilla Research Laboratory, Ahmedabad for providing experimental animals, Dr. Hardik Gandhi, Mr. Vishal Patel and Mr. Akash Patel for their help in animal handling as well as in biochemical estimations.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Sandwith NY: What Is Heterophragma Sulfureum Kurz? Kew Bulletin 1967; 21(1): 21-30.
- Khare CK: Indian medicinal plants. Springer Publication 2007; 308.
- Reddy RD: Forest Flora of Andhra Pradesh. Andhra Pradesh Forest Department, obtained from http://www. forests.ap.gov.in/Forest%20Flora%20of%20Andhra%20Pradesh/Family/Bignoniaceae.htm.
- Sasidharan N: Heterophragma quadriloculare (Roxb.) K. Schum, India Biodiversity Portal, obtained from http://indiabiodiversity.org/biodiv/species/show/229922.
- Satani BH, Surana VS, Patel RH and Mishra SH: Bioactive phytochemicals: Perspectives for modern medicine. Astral International Pvt. Ltd., New Delhi 2014; 2: 137-154.
- Barik R, Jain S, Qwatra D, Joshi A, Tripathi G and Goyal R: Anti-diabetic activity of aqueous root extract of Ichnocarpus frutescens in streptozotocin-nicotinamide induced type-II diabetes in rats. Indian Journal Pharmacology 2008; 40(1): 19-22.
- Bell RH and Hye RJ: Animal models of diabetes mellitus: physiology and pathology. The Journal of Surgical Research 1983; 35: 433-460.
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M and Ribes G: Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998; 47(2): 224-229.
- Rerup CC: Drugs producing diabetes through damage of insulin-secreting cells. Pharmacological Review 1970; 22: 485-518.
- Trinder P: Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of Clinical Biochemistry 1969; 6: 24-27.
- Wybenga DR, Pileggi VJ, Dirstine PH and Giorgio JD: Direct manual determination of serum total cholesterol with a single stable reagent. Clinical Chemistry 1970; 16(12): 980-984.
- Soumyanath A: Traditional medicines for modern times: antidiabetic plants. CRC Taylor and Francis Publication, United Kingdom 2006; 19.
- Gomes A, Vedasiromoni JR, Das M, Sharma RM and Ganguly DK: Antihyperglycemic effect of black tea (Camellia sinensis) in the rat. Journal of Ethnopharmacology 2001; 27: 243-275.
- Rajkumar L and Govindarajulu P: Increased degradation of dermal collagen in diabetic rats. Indian Journal of Experimental Biology 1991; 29: 1081-1083.
- Chatterjee MN and Shinde R: Textbook of medical biochemistry. Jaypee Brothers Medical Publishers, New Delhi 2002; 317.
- Swanston-Flatt SK, Day C, Bailey CJ and Flatt PR: Traditional plant treatment for diabetes: studies in normal and streptozotocin diabetic mice. Diabetologia 1990; 33: 462-464.
- Gupta R, Sharma AK, Dobhal MP, Sharma MC and Gupta RS: Anti-diabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. Journal of Diabetes 2011; 3: 29-37.
- Jang SM, Kim MJ, Choi MS, Kwon EY and Lee MK: Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism 2010; 59(4): 512-519.
- Lakshmi V, Mahdi AA, Ahmad MK, Agarwal SK and Srivastava AK: Anti-diabetic activity of lupeol and lupeol esters in streptozotocin-induced diabetic rats. Bangladesh Pharmaceutical Journal 2014; 17(2): 138-146.
- Satani BH, Surana V, Shah S and Mishra SH: Qualitative and quantitative phytochemical analysis of quadriloculare (Roxb.) K. Schum. leaves. Journal of Pharmacy and Applied Science 2016; 3(1): 18-25.
- Lally S, Owens D and Tomkin GH: Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: the relationship between the liver and intestine in control and streptozotocin diabetic rats. Metabolism 2007; 56(3): 430-8.
- Ginsberg HN: Very low-density lipoprotein metabolism in diabetes mellitus. Diab/Meta Rev 1987; 3(2): 571-89.
How to cite this article:
Satani BH, Surana VS, Shah SA and Mishra SH: Mushrooms: Evaluation of antidiabetic activity of Heterophragma quadriloculare (Roxb.) K. Schum. leaves. Int J Pharmacognosy 2017; 4(11): 378-83. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.4(11).378-83.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
378-383
607
1374
English
IJP
B. H. Satani*, V. S. Surana, S. A. Shah and S. H. Mishra
Maliba Pharmacy College, Uka Tarsadia University, Bardoli, Gujarat, India.
bhavik.satani@utu.ac.in
07 June 2016
21 August 2017
17 September 2017
DOI: 10.13040/IJPSR.0975-8232.IJP.4(11).378-83
01 November 2017