ESSENTIAL OIL OF CAMPOMANESIA AUREA: CHEMICAL COMPOSITION AND ANTI-NEOPLASTIC POTENTIAL IN-VITRO
HTML Full TextESSENTIAL OIL OF CAMPOMANESIA AUREAS: CHEMICAL COMPOSITION AND ANTI-NEOPLASTIC POTENTIAL IN-VITRO
H. O. Garcia * 1, L. A. Pacheco 2, J. G. Nuñez 1, G. C. Pinto 1, V. G. La Porta 1, G. L. Padilha 1, E. M. Ethur 2, L. Hoehne 2 and A. N. Bruno 1
Research Laboratory for Antineoplastic Compounds 1, Federal Institute of Education, Science and Technology of Rio Grande do Sul - Campus Porto Alegre, Porto Alegre, Brazil.
Graduate Program in Biotechnology, P. P. G Biotec 2, University of Vale do Taquari, Univates, Lajeado, Brazil.
ABSTRACT: Cervical cancer is a highly prevalent global disease among females and currently available treatments are nevertheless restricted and have substantial adverse effects. Plants may be a rich source of molecules with therapeutic potentials, such as Campomanesia aureas O. Berg, Myrtaceae, a native species from the South of Brazil with few data regarding its biological effects. In this study, we investigated the chemical composition and antineoplastic potential in-vitro of the essential oil (EO) of C. aureas leaves in human cervical cancer cells (SiHa), as well as its effect in the viability of non-tumoral cells (HaCat). CG-MS analysis reveals that the EO is rich in oxygenated sesquiterpenes and monoterpenes and MTT colorimetric assay shows that it significantly inhibits the viability of the SiHa cell line at different concentrations. Besides, the EO reduced the colony-forming capacity of the tumor cell line, and the ratio between the IC50 values of cell lines demonstrated a promising selectivity index for C. aurea EO. Therefore, the EO could be a potential source of new anticancer molecules.
| Keywords: |
Cervical cancer, Native plants, Campomanesia aurea, Essential oil
INTRODUCTION: Cancer is a major health problem worldwide, affecting 18.1 million people around the world in 2018, leading to the death of about 9.6 million people 1. Among females, cervical cancer ranks fourth in both incidence and mortality, just behind breast, colorectal, and lung cancer, as estimated by the International Agency for Research on Cancer 2. In Brazil, it is the third most common cancer type, with 16,340 new cases expected for 2020 3.
After the discovery of the causal relationship between infections caused by oncogenic subtypes of human papillomavirus (HPV) and cervical cancer, it was possible to establish health programs to prevent the disease and undertake early diagnosis of it, therefore achieving a decrease in incidence and mortality. Nevertheless, in countries and regions with low socioeconomic levels, it still remains prevalent.
Once the cervical carcinoma is diagnosed, the treatment of choice is radical hysterectomy with or without chemo-radiotherapy and radiotherapy, depending on the cancer’s stage 4. However, the recurrence of tumours after these most common treatments is up to 74% in the last stages 5. Chemo-radiotherapy for locally advanced uterine cervical cancer is associated with a typically poor pro-gnosis, with 5-year survival rates in approximately 60% of cases 6. Furthermore, the efficiency of radiotherapy to treat locally advanced cervical cancer is limited by the size of the tumor, because the doses required to treat large tumors exceed the limit of toxicity in normal tissue 7. Tese evidences make clear that new approaches are necessary to improve survival and upstand the quality of life of the patients in treatment. Many medicinal plants and their purified chemical constituents have shown beneficial therapeutic potential as alternatives for artificial additives or pharmacologically relevant agents. Among them, essential oils have gained popularity in the pharmaceutical industry due to a wide spectrum range of applications 8, 9. Essential oils are volatile plant secondary metabolites and may represent a rich and complex source of molecules with biological activity 10.
Many of them, especially the ones among Myrtaceae species, have been successfully used in folk medicine for different purposes; moreover have biological properties proven scientifically, such as antimicrobial 11, antioxidant 12, anti-inflammatory 13 and larvicidal 14 properties. The Campomanesia Ruiz & Pav is a well-defined genus of Myrtaceae and occurs in South America, more specifically in Southern Brazil, from the state of Rio Grande do Sul to Paraná, Argentina, Paraguay and Uruguay 15. Campomanesia aurea O. Berg species is popularly known in Brazil as "guabiroba-do-campo" or "araçá-rasteiro".
Previous studies that evaluated the chemical composition of the essential oil (EO) from some Campomanesia species demonstrated mono and sesquiterpenes as majority constituents in these oils 16. Since few data are found in the literature on C. aurea, the present study aims to gather information about this vegetable specie, studying the chemical composition and the in-vitro anti-neoplastic and cytotoxic potential of EO from leaves of C. aurea.
MATERIALS AND METHOD:
Culture Medium and Chemicals: Gentamicin, amphotericin B, and fetal bovine serum (FBS) were purchased from Gibco (Gibco BRL, Grand Island, NY). Dulbecco’s modified Eagle’s medium (DMEM), trypsin/EDTA solution, Trypan Blue dye, and MTT (3-[4, 5-dimethylthiazol - 2-yl] - 2, 5-diphenyl tetrazolium bromide) were purchased from Sigma Aldrich (St. Louis, MO, USA). All other chemicals and solvents used were of analytical grade.
Plant Material: Leaves of C. aurea were collected in São Francisco de Assis (29°33’00” S, 55°07’51” W). Identification was done by Botanist Prof. Dr. E. M. de Freitas. A specimen of the plant material was archived in the herbarium of the Museum of Natural Sciences (Museu de Ciências Naturais) of the University of Vale do Taquari - RS - Brazil, under the code HVAT 5093.
Essential Oil Extraction and Chemical Identification: The EO from C.aurea was extracted by hydrodistillation using a modified Clevenger apparatus. A quantity of 150 g of leaves was added to water at a proportion of 1:20 and boiled at 100 °C for 3 h 30 min. EO was separated by gravity, dried with anhydrous sodium sulfate, and kept in amber flasks under refrigeration until further chemical or biological examinations. Samples of EO were analyzed by gas chromate-graphy coupled to mass spectrometry (GC-MS) 17, at the Instrumental Analysis Laboratory, Food Processing Development Centre-FPDC, University of Vale do Taquari. The analysis was performed on a Shimadzu GC2010 Plus system, comprising a model AOC-5000 Plus auto-injector and a model QP2110 Ultra mass detector, using a Restek Rtx®-5MS fused silica capillary column (30 m x 0.25 mm i.d.; 0.25 μm film thickness).
The chromate-graphic conditions were: carrier gas - helium at a flow rate of 1.00 mL/min; oven temperature - initially at 50 °C and increased at 4 °C / min to 290 °C; injector temperature – 240 °C; injection mode - split with 1:20 ratio and 3 mL/min purge; MS interface temperature - 280 °C; ion source temperature - 260 °C; ionization energy - 70 eV. Oil samples (15 mg) were dissolved in 1.5 mL of purified ethyl acetate, and aliquots of 1μL were injected for analysis. GC analysis with flame ionization detection (FID) was carried out using an Agilent J&W HP-5MS column (30 m x 0.25 mm i.d.; 0.25 µm film thickness) with helium as a carrier gas, a FID temperature of 260 °C and an oven temperature program as described for the GC-MS procedure. Separated components were initially identified by their Kováts retention indices (RI), determined by a series of n-alkanes as a reference. Their identities were confirmed by comparison of the mass spectral data with those obtained using pure standards with values quoted in the literature 18and data stored in the Wiley 8 and NIST11 spectral libraries of the analytical system. The relative composition of the oils was calculated using the peak areas (uncorrected for specific response factors) of the separated components.
Cell lines Maintenance: SiHa (HPV 16-positive) human cervical carcinoma cells were obtained from American Type Culture Collection (ATCC - Rock-Ville, MD) and immortalized human keratinocytes, HaCat, were kindly donated by Dr. Luisa L. Villa (ICESP, School of Medicine, University of São Paulo) and Dr.Silvya S. Maria-Engler (Faculty of Pharmaceutical Sciences, University of São Paulo). Both cell lines were maintained in DMEM plus 10% FBS, 25 μg.mL-1gentamicin, and 0.5 μg. mL-1 amphotericin B. Cell cultures were kept at 37 °C in 5% CO2 atmosphere.
Cell Viability Analysis: The viability of SiHa and HaCat cell lines exposed to different concentrations of the EO from C. aurea was assessed by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) colorimetric assay 19. In order to do that, cells were seeded in 96-well plates (2.8 × 10³ cells / well) and placed at 37 °C in 5% CO2 atmosphere until completed cell adhesion. The culture medium was then aspirated and cells treated with the EO (0.01, 0.02, 0.03, 0.04, 0.05, 0.1, 0.2, 0.3, 0.5, 1.0 μg.mL-1) solubilized in DMEM medium with propylene glycol (Propane-1, 2-diol).
Cells incubated only in DMEM, and propylene glycol at the same final concentrations served as a control (untreated cells) and vehicle control, respectively. Following the 24 h treatments, MTT (0.5 mg. mL-1 in DMEM) was added to the wells and the plates were incubated for 3 h and 30 min at standard conditions of temperature and CO2 atmosphere.
MTT solution was removed, and the formazan crystals originated from the reduction of MTT by mitochondrial succinate dehydrogenase, were dissolved with dimethyl sulfoxide (DMSO). Well’s optical density was quantified at 545 and 630 nm using Spectra Max M2 Microplate Reader (Molecular Devices). The results were expressed as a percentage of control, which was considered as 100% of cell viability. The half-maximal inhibitory concentrations (IC50) values were calculated from log dose-response curves using GraphPad Prism 5 software.
Clonogenic Assay: In order to assess the ability of human cervical carcinoma single cells to grow into a colony after removal of treatment, we performed a clonogenic assay 20. SiHa cells were seeded in 24-well plates (2.8 × 104 cells/well) and, after adhesion, subconfluent cultures were treated with the EO from C. aurea at IC50 (0.03 μg. mL-1), DMEM, and DMEM with vehicle (propylene glycol) at the same final concentration for 24 h. Cells were then washed with phosphate-buffered saline (PBS), trypsinized, counted in hemo-cytometer, replatedin 24-well plates at low density (120 cells/well) and incubated for 5 days. The colonies were fixed with methanol, stained with violet crystal dye (0.5 mg. mL-1) and counted manually using a stereomicroscope. Results were expressed as survival fraction, which was obtained by dividing the number of colonies that arise after treatment by the number of cells seeded and plate efficiency (PE: number of colonies formed by untreated cells/number of cells seeded), multiplied by 100.
Statistical Analysis: Results were expressed as means and standard deviation (SD) from at least three independent experiments performed in triplicate. Data were analyzed using a one-way analysis of variance (ANOVA) followed by the Tukey test using the GraphPad Prism 5 (San Diego, USA, 2007). Statistical differences were considered significant when the p-value was < 0.05.
RESULTS:
Yield of Essential Oil and its Chemical Com-position: The yield of the EO from C. aurea was 4.44%. Twenty-nine compounds were found in the EO, and twenty-seven could be properly identified, corresponding to 93% of the total.
The EO showed a high content of monoterpenes (55.6%), of which 30.1% were hydrocarbon mono-terpenes, and 25.5% were oxygenated mono-terpenes. Oxygenated sesquiterpenes represented 28.7% of the content, and sesquiterpenes hydrocarbons were the minority group (12, 6%). The most representative molecule was the oxygenated sesquiterpene α-Cadinol (10, 72%) and the most representative’s monoterpenes were p-Cymene (8.3%) and α-Pinene (6.8%) Table 1.
TABLE 1: CHEMICAL COMPOSITION OF THE ESSENTIAL OIL OBTAINED FROM THE LEAVES OF CAMPOMANESIA AUREA O. BERG (EO)
| No | Component | KI expa | KI litb | Relative Percentage (%) in EO |
| 1 | α-Thujene | 936 | 930 | 1.59 |
| 2 | α-Pinene | 943 | 939 | 6.80 |
| 3 | β -Pinene | 980 | 979 | 4.85 |
| 4 | p-Cimene | 1024 | 1024 | 8.33 |
| 5 | Limonene | 1028 | 1029 | 2.00 |
| 6 | (1,8)-Cineole | 1031 | 1031 | 5.13 |
| 7 | γ-Terpinene | 1057 | 1059 | 3.03 |
| 8 | cis-linalol oxide | 1072 | 1072 | 0.41 |
| 9 | p-Menta-2,4(8)-diene | 1088 | 1088 | 3.46 |
| 10 | Linalool | 1100 | 1096 | 6.77 |
| 11 | Terpinen-4-ol | 1178 | 1177 | 4.85 |
| 12 | p-Cimen-8-ol | 1186 | 1182 | 0.99 |
| 13 | α-Terpineol | 1192 | 1188 | 7.38 |
| 14 | (E)-Caryophillene | 1420 | 1419 | 0.53 |
| 15 | α-Humulene | 1454 | 1454 | 0.67 |
| 16 | allo-Aromadendrene | 1462 | 1460 | 2.38 |
| 17 | α-Muurolene | 1501 | 1500 | 1.24 |
| 18 | γ-Cadinene | 1515 | 1513 | 1.80 |
| 19 | δ-Cadinene | 1525 | 1523 | 5.95 |
| 20 | (E)-Nerolidol | 1564 | 1563 | 1.11 |
| 21 | Palustrol | 1569 | 1568 | 0.60 |
| 22 | Spathulenol | 1579 | 1578 | 4.20 |
| 23 | n.i.c | 1585 | 2.42 | |
| 24 | n.i.c | 1600 | 0.71 | |
| 25 | Ledol | 1606 | 1602 | 1.25 |
| 26 | 1-epi-Cubenol | 1631 | 1628 | 1.53 |
| 27 | epi-α-Muurolol | 1645 | 1642 | 7.92 |
| 28 | α-Muurolol | 1649 | 1646 | 1.38 |
| 29 | α-Cadinol | 1658 | 1654 | 10.72 |
| Total identified | 96.87 | |||
| total hydrocarbon monoterpenes | 30.06 | |||
| total oxygenated monoterpenes | 25.53 | |||
| total hydrocarbon sesquiterpenes | 12.57 | |||
| total hydrocarbon sesquiterpenes | 28.71 | |||
Experimental Kovats retention index; b Literature Kovats retention index (Adams, 2007); c not identified
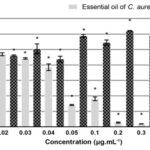
Effect on Cell Viability and Selective Index (SI): MTT assay is a well-established method based on the ability of cells with active metabolism to convert MTT salt into a purple-colored product. We assessed the viability of human cervical cancer cells (SiHa) and human keratinocyte cells (Haca T) after 24 h treatments with the EO from C. aurea at different final concentrations, ranging from 0.01 to 1 μg.mL-1 Fig. 1 and 2. All the concentrations tested, except 0.02 μg. mL-1, significantly decreased the viability of the tumor cells in relation to control cells. This inhibition varied between 7% and 97% Fig. 1, with a IC50 calculated of 0.045 μg.mL-1.
FIG. 1: EFFECT OF 24 H TREATMENT WITH DIFFERENT CONCENTRATIONS OF ESSENTIAL OIL OF C. AUREA ON THE VIABILITY OF CERVICAL CANCER CELL LINE (SIHA) AND VEHICLE (PROPYLENE GLYCOL). Data shows mean and standard deviation of at least three independent experiments performed in triplicate. *p<0,05 (one-way ANOVA, followed by Tukey’s Test).
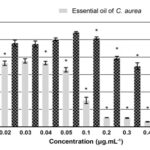
The treatments also decreased significantly the viability of the non-tumour cells (HacaT) between 12.0 % and 94.2%, except at 0.03 μg.mL-1 Fig. 2. The IC50 value for the EO in these cells was of 0.06 μg.mL-1, higher than the one calculated for the human cervical cancer cells.
Besides, it was possible to observe a different profile in non-tumour cells once it was necessary higher concentrations to achieve the same percentage of cell viability inhibition.
In order to obtain the selective-index (SI) from EO, IC50 values in non-tumour cells (0.06 μg. mL-1) were divided by IC50 values in cancer cells (0.045 μg. mL-1). The calculated SI for the EO was 1, 33, and values higher than 1.00 are already considered therapeutically promising 21.
FIG. 2: EFFECT OF 24 H TREATMENT WITH DIFFERENT CONCENTRATIONS OF ESSENTIAL OIL OF C. AUREA ON THE VIABILITY OF IMMORTALIZED HUMAN KERATINOCYTES CELL LINE (HACAT) AND VEHICLE (PROPYLENE GLYCOL). Data shows mean and standard deviation of at least three independent experiments performed in triplicate. *p<0, 05 (one-way ANOVA, followed by Tukey’s Test).
Effect in the Clonogenic Ability of Cervical Cancer Cells: The clonogenic assay allows us to determine the ability of a single cell to undergo unlimited division to form a large colony 22.
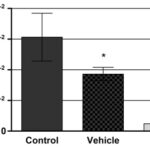
The clonogenic assay results can be seen in Fig. 3 and 4 and show that the number of colonies that arose after 24 h treatment with the EO from leaves of C. aurea (at 0.045 μg.mL-1) has significantly decreased (94%) in relation to control.
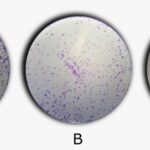
The loss of clonogenic capacity in the treated tumour cells can also be seen qualitatively in Fig. 4, where we show the pictures of fixed and coloured wells.
FIG. 3: EFFECT OF VEHICLE (PROPYLENE GLYCOL) AND C. AUREA’S ESSENTIAL OIL (IC50 = 0.045 UG/ML) ON CLONOGENIC CAPACITY OF SIHA CELLS AFTER 24 H TREATMENT. Surviving Fraction represents the number of colonies formed after treatment divided by the number of cells plated versus plating efficiency. *p<0, 05 (one-way ANOVA, followed by Tukey’s Test).
FIG. 4: REPRESENTATIVE PICTURES OF THE COLONIES FORMED BY SIHA CELLS AFTER DIFFERENT TREATMENTS. Control (A), Vehicle (B) and IC50 (0.045 μg.mL-1) (C).
DISCUSSION: In this study, we evaluated the composition and antineoplastic potential of the EO from the leaves of C. aurea collected in southern Brazil (state of Rio Grande do Sul). Despite being a Myrtaceae and belonging to a well-established genus, there is few scientific information about its EO composition and biological effects. EO as well as other plant-derived products has been historically used for the treatment and prevention of various disorders in popular medicine. Recently, these products have gained interest due to their complex composition and biological effects, which have been increasingly studied. In plants, stationary organisms, EOs are a part of the chemistry defense against external threats, such as herbivores. It is well known that the production of the EO depends on many factors, including the genetic and physiological state of the plant, as well as abiotic factors, such as environmental conditions 23.
The majority compound of the EO in this study was α-Cadinol (10.72%), as shown in Table 1. This oxygenated sesquiterpene molecule is already reported to have selective cytotoxic activity against human cancer cell lines such as colon adeno-carcinoma (HT-29) 24, breast adenocarcinoma (MCF-7) 25, lung adenocarcinoma (A-549) and human hepatocellular carcinoma (J5) 26. Moreover, oxygenated sesquiterpenes, which represent 28.7% of the total EO from C. aurea, may be the main molecules cooperating with the cytotoxic effect observed in the human cervical cancer cells, rather than the hydrocarbonate mono and sesquiterpenes. Paduch et al 27 have showed that the addition of alcohol or ketone radicals to terpenes enhances their antitumor activity.
Regardless of being rich in oxygenated sesqui-terpenes, the EO in this study exhibits a good monoterpene fraction (55.6%), which is a typical content profile in relation to what is already described in the literature about the species. Limbergeret al 28 studied the composition of EO from leaves of four Campomanesia species collected in Southern Brazil, showing that the monoterpene fraction was well-represented only in C. aurea (40.3%). Although, in the referred study, α-Pinene was found in C. aurea’s EO in higher amounts (16.5%), and p-Cymene was not detected. It illustrates the variation in oil content of the same plant in different geographic locations. Environmental factors have been identified as the main responsible for the fluctuation of secondary metabolite contents in plants 29. Monoterpenes are a group of molecules that represent an important source of biologically and pharmacologically active compounds 30 whose structures may help to design and synthesize new and low-toxicity drugs.
The monoterpene p-Cymene, which represents 8.33% of the EO, is already known for several biological activities, such as antioxidant, anti-microbial and antinociceptive properties 31. In fact, a pre-clinical study conducted by Santos et al., 32 have shown that this molecule exerts antinociceptive effects on oncological pain, modulating calcium channel currents. Jaafari et al., 33 showed that the monoterpene carvacrol, a derivative of p-Cymene, exhibits a large cytotoxic effect in mastocytoma cells. Other studies also show that carvacrol causes cell cycle arrest and induces apoptosis in human breast adenocarcinoma (MCF-7 cells) and in some types of leukemia cells 34. α-Pinene, another significant monoterpene found in the EO, equally shows a wide range of biological activities, for instance antimicrobial 35 and gastrop-rotective 36. It can also inhibit human prostate cancer cell growth, inducing apoptosis in-vitro and in-vivo 37, and exerts inhibitory activity on human colon tumor in-vitro by suppressing mitochondrial enzyme activity and destabilizing the membrane 38. Despite the fact that the isolated monoterpenes may be responsible for cytotoxic and antiproliferative effects, we must consider that the EO is a complex mixture of different chemical compounds and there may be cooperative, synergistic and/or antagonistic interactions among them.
An interesting study performed by Wang et al., 39 assessed the antibacterial and anticancer activity of Rosmarinus officinalis L. EO and three of its main isolated compounds (1, 8-cineole, α-pinene and β-pinene), and concluded that the total EO have higher cytotoxic effect than the isolated compounds against two human ovarian cancer cell lines (SK-OV-3, HO-8910) and one human papillomavirus-related endocervical adenocarcinoma cell line (Bel-7402). The same authors have previously observed that the same Rosmarinus officinalis total EO shows stronger antioxidant activity than its isolated compounds, concluding that the minor molecules may make a significant contribution to the biological effects 40. In our results, it was possible to observe inhibitory effects on the viability of tumor cells at very low concentrations, which is reflected in an equally low IC50 value (0.045 μg. mL-1). These results are interesting since drugs that are effective at low concentrations can present limited adverse effects on non-tumor cells.
Besides, according to the American National Center Institute, only natural compounds with IC50 values lower than 30 μg. mL-1 against tumor cell lines constitute promising agents for development of potential anticancer drugs 41. Additionally, the difference between the IC50 values between tumor and non-tumor cells after EO treatment resulted in a selectivity index higher than one, which is considered therapeutically promising because it means that this compound is more cytotoxic to the tumor cell line than to the normal cell line.
Furthermore, EO from C. aurea reduced markedly the capacity of colony-forming in tumor cell after 24 h of treatment. It is well documented that the capacity of colony formation is essential for cells to grow and expand in a tumor microenvironment, and the clonogenic assay is a significant method to determine the fraction of seeded cells that retain the capacity to produce colonies 20, 42, 43. For this reason, the clonogenic assay is useful to determine the effectiveness of cytotoxic agents and the tumorigenicity in-vivo and a reduction of clonogenic capacity is related to a decrease in tumor growth and cancer progression 20. Usually, cells grown in colonies are less sensitive to cytotoxic agents than cells grown in a monolayer because of the larger surface they expose to the drug, compared to the limited drug penetration in the colonies 44. Then, it is important to consider that in this study the EO was efficient in both monolayer and colony cell culture conditions.
Regarding the mechanism of action of the EO in tumor cells, it is known that the effects induced by antitumor agents may be metabolic or reproductive 45, however, although EO has reduced the viability of tumor cells as well as the clonogenic capacity of these cells, additional studies are needed to verify whether this EO confers toxicity or antiproliferative effects. Additionally, natural compounds may inhibit cancer cell growth by different mechanisms, such as cell cycle arrest, enhancement of gap junctional communication and induction of apoptosis, and these mechanisms are related to clonogenic capacity, invasion, and migration of tumor cells 46. It is also known that these cellular processes are associated with tumorigenesis and metastasis 47. Considering the importance of developing novel anticancer agents, we believe that the EO from C. aurea deserves further, investi-gation, once it may be a source of bioactive compounds with antitumor potential.
CONCLUSION: Chemical characterization by GC-MS showed that the most representative molecule in essential oil from C. aurea’s leaves is α-Cadinol (10, 72%) and that it has high content of monoterpenes. The ability of the oil to decrease viability and colony-forming capacity of human cervical cancer cells with a good selectivity index shows that the oil may be a promising source of new anticancer molecules and deserves further investigation concerning to its effects and mechanisms.
ACKNOWLEDGEMENT: The authors are grateful to Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Sul and Universidade do Vale do Taquari (Univates) for financial support.
CONFLICTS OF INTEREST: The authors declare that there is no conflict of interest.
REFERENCES:
- World Health Organisation. Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Press Release. 2018; 263: 13-15.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394-24.
- Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2020: Incidência de Câncer No Brasil 2019.
- Hernández VMV: End of the Minimal Invasion Surgery in Cervical Cancer. Clin Oncol 2018; 3: 3-5.
- Kumar N: Cervical Cancer; a Nightmare for Womanhood: Review of Recent Advances Naina. Womens Heal Gynecol 2016; 2(2): 30-34.
- Eifel P, Moughan J, Erickson B and Iarocci T: Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix: a patterns of care study. Int J Radiol Oncol Phys 2004; 60(4): 1144-53.
- Fletcher GH: Textbook of Radiology. Lea and Febiger, 2nd edition 1973.
- Kalemba D and Kunicka A: Antibacterial and Antifungal Properties of Essential Oils. Curr Med Chem 2005; 10(10): 813-29.
- Lang G and Buchbauer G: A review on recent research results (2008-2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr J 2012; 27(1): 13-39.
- Erich Schmidt: Production of essential oils. Handbook of Essential Oils Science Technology and Applications CRC Press 2010: 83-19.
- Silva CJ, Barbosa LA and Demuner AJ: Chemical composition and antibacterial activities from the essential oils of Myrtaceae species planted in Brazil. Quim Nov 2010; 33(1): 104-08.
- Carneiro NS, Alves CCF and Alves JM: Chemical composition, antioxidant and antibacterial activities of essential oils from leaves and flowers of Eugenia klotzschiana Berg (Myrtaceae). An Acad Bras Cienc 2017; 89(3): 1907-15.
- Pietrovski EF, Magina MDA and Gomig F: Topical anti-inflammatory activity of Eugenia brasiliensis (Myrtaceae) leaves. J Pharm Pharm 2008; 60(4): 479-87.
- Neves IA, Rezende SRF, Kirk JM, Pontes EG and Carvalho MG: Composition and larvicidal activity of essential oil of Eugenia candolleana (Myrtaceae) against Aedes aegypti. Rev Virtual Quim 2017; 9(6): 2305-15.
- Lima DF, Goldenberg R and Sobral M: O gênero Campomanesia (Myrtaceae) no estado do Paraná, Brasil. Rodriguesia 2011; 62(3): 683-93.
- Amaral do W, Deschamps C, Bizzo HR, Pinto MAS, Silva LE and Biasi LA: Teor e composição química do óleo essencial de espécies nativas da família Myrtaceae nos Campos Gerais da Floresta Atlântica do Estado do Paraná. Rev Bras Plantas Med 2016; 18(14): 890-98.
- Kauffmann C, Pacheco LA, Buhl B and Scheibel T: Avaliação da atividade leishmanicida in-vitro de espécies da família Myrtaceae, nativas do sul do Brasil. Rev Destaques Acadêmicos 2017; 9(3): 246-58.
- Sparkman OD: Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy Robert P. Adams. J Am Soc Mass Spectrom 2005; 16(11): 1902-03.
- Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1-2): 55-63.
- Franken NAP, Rodermond HM, Stap J, Haveman J and van Bree C: Clonogenic assay of cells in-vitro. Nat Protoc 2006; 1(5): 2315-19.
- Callacondo-riva D, Quispe-mauricio A, Lindo-gamarra S and Vaisberg AJ: Actividad citotóxica del extracto etanólico de Gnaphalium. Rev Peru Med Exp Salud Publica 2008; 25(4): 380-85.
- Munshi A, Hobbs M and Meyn RE: Clonogenic Cell Survival Assay. Chemosensiti Totowa 2005; 110: 21-29.
- Lee YL and Ding P: Physiological Production of Essential Oil in Plants - Ontogeny, secretory structures and seasonal variations. Review Pert J Sch Res Revies 2016; 2(1): 0-10.
- He K, Zeng L, Shi G, Zhao GX, Kozlowski JF and McLaughlin JL: Bioactive compounds from Taiwania cryptomerioides. J Nat Prod 1997; 60(1): 38-40.
- Chang S, Wang DS, Wu C, Shiah S, Kuo Y and Chang C: Cytotoxicity of extractives from Taiwania cryptomerioides heartwood. Phytochemistry 2000; 55: 227-32.
- Su YC, Hsu KP, Wang EIC and Ho CL: Composition, in-vitro cytotoxic, and antimicrobial activities of the flower essential oil of diospyros discolor from Taiwan. Nat Prod Commun 2015; 10(7): 1311-14.
- Paduch R, Trytek M and Król SK: Biological activity of terpene compounds produced by biotechnological methods. Pharm Biol 2016; 54(6): 1096-07.
- Limberger RP, Apel MA, Sobral M, Moreno PRH, Henriques AT and Menut C: Chemical composition of essential oils from some Campomanesia species (Myrtaceae). J Essent Oil Res 2001; 13(2): 113-15.
- Waterman PG and Mole S: Entrinsic factors influencing production of secondary metabolites in plants. Insect-Plant Interactions, Boca Raton, 1st edition, 1989: 107-34.
- Salakhutdinov NF, Volcho KP and Yarovaya OI: Monoterpenes as a renewable source of biologically active compounds. Pure Appl Chem 2017; 89(8): 1105-17.
- Marchese A, Arciola CR and Barbieri R: Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials (Basel) 2017; 10(8): 1-15.
- Santos WBR, Melo MAO and Alves RS: P-Cymene Attenuates Cancer Pain via Inhibitory Pathways and Modulation of Calcium Currents. Phyto 2019; 61: 152836.
- Jaafari A, Tilaoui M and Mouse HA: Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Brazi J Pharma 2012; 22(3): 534-40.
- Sobral MV, Xavier AL, Lima TC and De Sousa DP: Antitumor activity of monoterpenes found in essential oils. Sci World J 2014: 1-35.
- Silva ACR, Lopes PM, Azevedo MMB, Costa DCM, Alviano CS and Alviano DS: Biological Activities of α-Pinene and β-Pinene Enantiomers. Molecules 2012; 17: 6305-16.
- Pinheiro MA, Magalhães R and Torres D: Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn Mag 2015; 11(41): 123.
- Zhao Y, Chen R, Wang Y and Yang Y: Α-Pinene Inhibits Human Prostate Cancer Growth in a Mouse Xenograft Model. Chemotherapy 2018; 63(1): 1-7.
- Trytek M, Paduch R and Pięt M: Biological activity of oxygenated pinene derivatives on human colon normal and carcinoma cells. Flavour Fragr J 2018; 33(6): 428-37.
- Wang W, Li N, Luo M, Zu Y and Efferth T: Antibacterial activity and anticancer activity of Rosmarinus officinalis essential oil compared to that of its main components. Molecules 2012; 17(3): 2704-13.
- Wang W, Wu N, Zu YG and Fu YJ: Antioxidative activity of Rosmarinus officinalis essential oil compared to its main components. Food Chem 2008; 108(3): 1019-22.
- Suffness M and Pezzuto JM: Assays related to cancer drug discovery. Methods in Plant Biochemistry: Assays for Bioactivity. Academic Press 1990: 71-33.
- Toomeh D, Gadoue SM, Yasmin-karim S, Singh M and Shanker R: Minimizing the potential of cancer recurrence and metastasis by the use of graphene oxide nano- flakes released from smart fiducials during image- guided radiation therapy. Phys Medica 2018; 55: 8-14.
- Zhang W, Chen L and Xiang H: Knockdown of GGCT inhibits cell proliferation and induces late apoptosis in human gastric cancer. BMC Biochem 2016: 1-7.
- Brown MM and Attardi LD: The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 2005; 5(3): 231-37.
- Roper PR and Drewinko B: Comparison of in Vitro Methods to Determine Drug-induced Cell Lethality. Cancer Res 1976; 36: 2182-88.
- Wang SJ, Zheng CJ and Peng C: Plants and cervical cancer: an overview. Expert Opin Investig Drugs 2013; 22(9): 1133-56.
- Zhang T, Li J and Dong Y: Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion. Breast Cancer Res Treat 2012; 135: 445-58.
How to cite this article:
Garcia HO, Pacheco LA, Nuñez JG, Pinto GC, Porta VGL, Padilha GL, Ethur EM, Hoehne L and Bruno AN: Essential oil of Campomanesia aurea: chemical composition and antineoplastic potential in-vitro. Int J Pharmacognosy 2020; 7(12): 361-68. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(12).361-68.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
361-368
859
962
English
IJP
H. O. Garcia *, L. A. Pacheco, J. G. Nuñez, G. C. Pinto, V. G. La Porta, G. L. Padilha, E. M. Ethur, L. Hoehne and A. N. Bruno
Research Laboratory for Antineoplastic Compounds, Federal Institute of Education, Science and Technology of Rio Grande do Sul - Campus Porto Alegre, Rua Coronel Vicente, Centro Histórico, Porto Alegre, Brazil.
helana.garcia@poa.ifrs.edu.br
27 August 2020
23 September 2020
22 November 2020
10.13040/IJPSR.0975-8232.IJP.7(12).361-68
31 December 2020