EFFECTS OF DEFATTED MORINGA OLEIFERA SEED ON SKELETAL MUSCLE OF PROTEIN ENERGY MALNOURISHED RATS
HTML Full TextEFFECTS OF DEFATTED MORINGA OLEIFERA SEED ON SKELETAL MUSCLE OF PROTEIN ENERGY MALNOURISHED RATS
N. Charles David *, Okoliko Micheal Victor, Mathias Akoji Godwin, Ekwoba Lucky, F. Nwobodo Chiamaka, Ozonkwo Mary, B. Olorunfemi Oyewole, O. Okeme Theresa , E. Omeje Helen, O. Gift Rebecca and Muritadoh Toheeb
Department of Biochemistry, Olusegun Agagu University of Science and Technology, Okitipupa, Nigeria.
ABSTRACT: Background: Malnutrition in form of protein energy malnourishment, is a range of pathological conditions arising from coincident lack of protein and/or energy in varying proportions which can results in muscle wasting and degeneration. Aim: The study is aimed to investigate the effects of defatted Moringa oleifera seed on skeletal muscle of Protein Energy Malnourished rats and to assess its effect on enzyme markers: AST, ALT, Total protein, Albumin, Ca2+ ATPase and Na+/K+ ATPase. Methods: 16 white albino rats of waster strain (Rattus novergicus) were initially divided into ‘A’ and ‘B’ groups; Group A rats served as positive control and was administered normal growers’ feed while Group ‘B’ rats were administered with low protein based diet to induce muscle wasting for 21 days. After the period of malnourishment, group B was further divided into 3 groups: C, D, E. Group C was sacrificed which served as negative control while group D and E were fed with recovery diet containing 20% M. oleifera based diet and 20% fish meal based diet respectively for another 21 days. Results: Upon the introduction of recovery diet, the results show that there was an increase in the activities of ALT, AST, Na+/K+ and Ca2+ ATPases as well as the total protein and albumin when compared with the control. Conclusion: M. oleifera leaf-based diet proves to be a sustainable replacement for food protein in the diet.
Keywords: Protein energy malnutrition, Moringa oleifera, Enzyme markers
INTRODUCTION: We know that calcium ion maintains the cellular balance between protein synthesis and protein degradation in the muscle cells.
Generally, altered protein metabolism in the muscle cells leads to muscle degeneration as well as various disease conditions including micro-nutrient deficiency, over-weight, infections, under-nutrition etc. 22 which can greatly affect calcium ion levels, ATPase activity, oxidation/reduction state of the cell and cause oxidative stress. Calcium adenosine triphosphatase (Ca2+ - ATPase) is important for maintaining the overall health of the muscle cells, however inhibition of Ca2+ - ATPase prevent the pumping of calcium ion, resulting in muscle wasting and degeneration of muscle tissue 22. In addition to calcium homeostasis in the muscle cells, Na+/K+-ATPase have been known to maintain the resting potential, and regulate cellular volume of the cell. It also functions as a signal transducer/integrator to regulate MAPK pathway, and reactive oxygen species in cells 13.
Malnutrition is the outcome of an unbalanced, inadequate, or excessive intake of nutrients and energy. The phrase covers both protein energy malnutrition (PEM) and all of the previously listed disease symptoms. PEM is a kind of malnutrition that is described as a set of clinical symptoms brought on by simultaneous protein and/or energy deprivation in varied ratios. The severity of the ailment ranges from moderate to severe, depending on the form 12.
The three most prominent types of PEM are Kwashiorkor (protein malnutrition predominate), Marasmus, and Marasmic Kwashiorkor (major protein deficiency and strong calorie insufficiency signs present; occasionally referred to as the most severe form of malnutrition) 21.
The "Miracle tree," Moringa oleifera (family: Moringaceae), is a commonly used medicinal plant that possesses a number of pharmacological and health-improving qualities 17. Due to the fact that every part of the tree is edible, M. oleifera has a range of uses. It's noteworthy to mention that consumption of this plant has been demonstrated to drastically boost the intake of certain critical minerals and phytochemicals that are known to promote human health 28.
Due to its ease of growing and the dispersion of phytochemicals in all of the plant's components, including the leaves, flowers, pods, and seeds 18-30 identify M. oleifera as a wonderful remedy to combat malnutrition. According to studies by 3, 10, 16, 17, 26 and 31 vitamins, polyphenols, carotenoids, phytosterols, and tocopherols are the most prevalent ingredients. From both in-vivo and in-vitro research, the literature reveals that this plant has more than 20 different pharmacological effects 27.
The plant's proximate analysis demonstrated a high percentage production of protein and carbs that are appropriate for food fortification and have significant potential as nutritional supplements 4. Therefore, the goal of this study is to examine into the effects of defatted Moringa oleifera seed on the skeletal muscle of protein-energy deficient rats.
MATERIALS AND METHODS:
Plant Materials: Moringa oleifera seed was bought from Kogi State University farm Anyigba. The seeds were taken from the seed coat and dried at 600C then crushed.
A known weight of the pulverized seeds was measured into a savette paper wrapped by the use of a white thread; it was thereafter maintained in a washed and dried conical flask. N-Hexane was added to the conical flask and shaken violently and permitted to stand for roughly three hours to defat adequately. The defatted seed was untied and put on a new savette paper for optimum drying at room temperature.
TABLE 1: COMPONENTS OF THE CONTROL AND TEST DIETS
| Diet Components | Protein energy malnutrition diet | Fish meal based recovery diet | Defatted Moringa oleifera seed based recovery diet |
| Defatted grounded Moringa oleifera seed | 40g | - | 100g |
| Grounded fish | - | 100g | - |
| Corn chaff | 530g | 470g | 470g |
| Vitaflash Amino WSP (vitamins-amino acids) | 30g | 30g | 30g |
| Vegetable oil | 50g | 50g | 50g |
| Sucrose | 350g | 350g | 350g |
*Vitaflash Amino WSP (vitamins-amino acids) composition per 1000gram: vitamin A, 10000000i.u; vitamin D3, 2000000i.u; vitamin E, 15000mg; vitamin K3, 2500mg; vitamin B1,1000mg; vitamin B2, 2000mg; vitamin B6, 2000mg; vitamin B12, 10000mcg; Folic acid,300mg; Ca-d-pantothenate, 7500mg; Nicotinic acid,20000mg; choline chloride,15000mg; vitamin C, 40000mg; DL-methionine, 50000mg; L-lysine, 50,000mg; Amino acids, 52,000mg.
Experimental Animals: Sixteen (16) female albino rats of waster strain (Rattus Novergicus) weighing between 57-120g were used for the study.
The rats were obtained from the animal house of the department of Biochemistry, Kogi State University, Anyigba, Kogi State. All the rats were fed with growers’ marsh and clean water for a week in the animal house of Biochemistry, Kogi State University, Anyigba for them to acclimatize prior to experimentation. They were kept in properly ventilated cages.
Animal Grouping: Sixteen albino rats (weighing 57-120g) were initially grouped into two groups of ‘A’ (made up of the four {4} positive control fed with growers’ feed) and ‘B’ (made up of the twelve rats fed with the Protein Energy Malnutrition diet).
At the end of the twenty-one (21) days of malnourishment four rats under ‘B’ were randomly selected and sacrificed with subsequent removal of their skeletal muscles to serve as the negative control (Group C).
The remaining eight rats under ‘B’ were grouped into ‘D’ and ‘E’ made up of four {4} rats each.
Group A (Positive Control): Fed with normal growers’ feed.
Group C (Negative Control): Fed only with Protein Energy Malnutrition diet.
Group D: fed with 20% M. oleifera seed based recovery diet.
Group E: fed with 20% fish meal based recovery diet.
At the end of the 21days of feeding the animals with the recovery diets, the rats were decapitated by cervical dislocation and were sacrifice.
Preparation of Tissue Homogenate: The rodents were killed by the dislocation of the cervical spine, and blood was obtained using a jugular puncture. For serum and hematological analysis, blood specimens were put into plain bottles and some into EDTA coated sample vials (to limit clotting). (250 mM sucrose, 10 mMtris, pH 7.4) was administered to quickly extract skeletal muscle from the hind limbs 2.
After that, serum was created by centrifuging the blood samples at 3000 rpm for 5 minutes 20. The buffer was utilised as the homogenizing medium to mix the skeletal muscle in an ice-cold mortar and pestle. Aliquots of the tissue homogenate suspension were stored in Eppendoff tubes and kept in the freezer. To achieve the maximal potential release of the enzymes, the homogenate was kept frozen overnight 19. The homogenate was then utilised for enzyme testing.
Biochemical Test: Using bovine serum albumin as the reference protein, the protein concentration in the tissue homogenates was assessed using the Biuret method, which was first described by 11.
The method provided by 8 was used to test the concentration of serum albumin. The method described by 25 was used to test the activities of aspartate transaminase (AST), alanine transaminase (ALT), and Na+/K+ ATPase in skeletal muscle tissue homogenate. Using the approach recommended by Bewaji (2004), Ca2+ -ATPase was assessed in the skeletal muscle tissue homogenate after four and eight weeks.
Analysis: All data are expressed as the mean of four (4) replicates. Statistical investigation of mean was performed by MS excel
RESULTS:
Animal Morphology: With the exception of the control group, the animals' weekly mean weight gradually decreased during malnutrition. When recovery diet treatments began, the situation was the opposite. The animals in Group A (control) developed well, with smoother skin fur, an oblong face, and tail covered in fur; no fur loss was noted in any place.
In contrast, the starved animals (Group B) demonstrated decrease of appetite, which may have contributed to the body fur loss, moon face development, unchanged head circumference, scaly tails, enlarged eyes, and muscle atrophy that were seen. The morphological modifications mentioned above improved over the course of the treatment.
Effects of Diets on Liver Function Indices (AST, ALT, Serum Albumin, Protein): As shown in Fig. 1, the blood concentration of AST, ALT, serum albumin, and protein was lowered in experimental rats after feeding with low protein iso-caloric diets. This serum concentration of these markers considerably increased after feeding the malnourished animals with the designed treatment meals.
FIG. 1: THE EFFECT OF ADMINISTRATION OF DEFATTED MORINGA OLEIFERA SEED, FISHMEAL FEED AND NORMAL GROWER’S FEED ON THE AST, ALT, SERUM ALBUMIN, AND PROTEIN LEVEL IN SKELETAL MUSCLE OF ALBINO RATS. MR= Moringa oleifera rich meal; FM= fish meal; PC= positive control; NC= negative control.
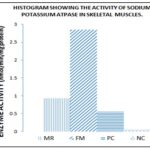
Calcium ATPase Activity: As shown in Fig. 2, the calcium ATPase activity of the malnourished rats had a rise in activities compared with the positive control However; ATPase activity in the Moringa oleifera treated group had great increase in activity after treatment
FIG. 2: THE EFFECT OF ADMINISTRATION OF DEFATTED MORINGA OLEIFERA SEED, FISHMEAL FEED AND NORMAL GROWER’S FEED ON CALCIUM ATPASE ACTIVITY IN SKELETAL MUSCLE OF ALBINO RATS. MR= Moringa oleifera rich meal; FM= fish meal; PC= positive control; NC= negative control.
Na+/K+ ATPase Activity: According to Fig. 3, the Na+/K+ ATPase activity was decreased in the experimental animals after feeding with low protein iso-caloric diets.
This blood concentration of Na+/K+ ATPase rose after feeding the impoverished animals with the planned therapeutic meals.
FIG. 3: THE EFFECT OF ADMINISTRATION OF DEFATTED MORINGA OLEIFERA SEED, FISHMEAL FEED AND NORMAL GROWER’S FEED ON NA+/K+ ATPASE ACTIVITY IN SKELETAL MUSCLE OF ALBINO RATS. MR= Moringa oleifera rich meal; FM= fish meal; PC= positive control; NC= negative control.
DISCUSSION AND CONCLUSION: Malnutrition, particularly Protein-Energy Malnutrition (PEM), can lead to different morphological changes such as edema, diarrhea, weight loss, hair loss, blindness, vulnerability to infections, and lower total protein levels 24.
The present research fits with these findings, suggesting that increased diet can restore these detrimental morphological alterations reported in test animals to some extent, though with different capacity. Patients with protein deficiency (Kwashiorkor) are known to have low hematological indicators, including AST, ALT, serum albumin, and protein levels 1, 6.
However, feeding rodents with a diet based on Moringa oleifera leaves, which are rich in β-carotene, protein, vitamin C, calcium, and potassium 29, indicates its potential as a sustainable substitute for dietary protein in rat feed. Moreover, the inclusion of M. oleifera leaf-based diet in this study restored liver indicators, such as blood albumin levels and AST/ALT activity, suggesting enhanced liver function. Following skeletal muscle degeneration induction, the activity of Ca2+ ATPase in the skeletal muscle of all groups reduced. However, animals fed with the M. oleifera leaf-based diet displayed higher resilience to the effects of starvation on enzyme function compared to other groups. The reduction in Ca2+ ATPase function during starvation can come from decreased enzyme production or inactivation, leading to hazardous high concentrations of Ca2+ within the cell and eventual cell death 7.
Conversely, the considerable increase in Ca2+ ATPase activity found in the M. oleifera leaf-based diet group could be attributable to enzyme activation or enhanced synthesis during treatment, showing the reversibility of the effect of malnourishment on this enzyme.
The Na+/K+-ATPase serves a key function in maintaining the concentration of ions across the plasma membrane, contributing to the resting membrane potential in cells. Studies have revealed that its levels are low in specific metabolic situations 9-23, which coincides with the findings of this investigation. Notably, animals fed with M. oleifera leaf-based diet and fish meal diet exhibited a significant increase in serum Na+/K+-ATPase activity after treatment, possibly indicating enzyme activation or increased synthesis during the treatment, suggesting the reversible nature of the effect of malnutrition on Na+/K+-ATPase. In conclusion, this research indicates that malnutrition-induced muscle degeneration considerably alters Ca2+ ATPases, Na+/K+-ATPase, AST, ALT, protein levels, and serum albumin in skeletal muscles. However, the adverse consequences are most adequately addressed by the M. oleifera leaf-based diet. The process by which M. oleifera leaf improves muscle degeneration induced by PEM might comprise improving the production and/or activity of the studied markers, thereby minimizing the impact of oxidative stress and possibly decreasing the loss of energy in the skeletal muscles. Thus, the M. oleifera leaf-based diet proves to be a sustainable replacement for dietary protein in the diet.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Adesola AO: The influence of severe protein deficiency (kwashiorkor) on gastric acid secretion in Nigerian children. The British Journal of Surgery 1968; 55(11): 866-866.
- Akanji M and Ngaha EA: Effect of repeated administration of berenil and urinary excretion with corresponding tissue pattern in rats. Pharmaceutical Toxicology 1989; 64: 272-275.
- Aly AA, Maraei RW and Ali HGM: Fatty acids profile and chemical composition of Egyptian Moringa oleifera seed oils. J Am Oil Chem’ Soc 2016; 93(3): 397-404.
- Bamishaiye EI, Olayemi FF, Awagu EF & Bamshaiye OM: Proximate and phytochemical composition of Moringa oleifera leaves at three stages of maturation. Advance Journal of Food Science and Technology 2011; 3(4): 233-237.
- Bewaji CO: Enzymes-Determination of the Adenosine Triphosphatase Activities in Rat Brain. Experimental Biochemistry. Klobex Academic Publishers 2004; 45-46.
- Bolarinwa AF, Oyebola DDO & Akindeinde GB: Effect of malnutrition on basal and induced gastric acid secretion. Nigerian J of Physiological Sciences 1991; 5: 144-148.
- Carafoli E: Calcium pump of the plasma membrane. Physiological Reviews 1991; 71(1): 129-153.
- Doumas BT, Watson WA and Biggs HG: Albumin Standards and the Measurement of Serum Albumin with Bromocresol Green. Clinica Chimica Acta 1971; 31: 87.
- Dufayet De La Tour D, Raccah D, Jannot MF, Coste T, Rougerie C & Vague P: Erythrocyte Na/K ATPase activity and diabetes: relationship with C-peptide level. Diabetologia 1998; 41: 1080-1084.
- Glover-Amengor M, Aryeetey R, Afari E and Nyarko A: Micronutrient composition and acceptability of Moringa oleifera leaf‐ fortified dishes by children in Ada‐ East district, Ghana. Food Sci Nutr 2017; 5(2): 317-323.
- Gornall AG, Bardawill CJ and David MM: Determination of serum proteins by means of the biuret reaction. Journal of Biological Chemistry 1949; 177: 751-766.
- Jee KO: Protein energy malnutrition: an overview. International Journal of Homoeopathic Sciences 2021; 5(1): 368-373.
- kasas AE, Farag IM, Darwish HR, Soliman YA, Nagar EE, Ibrahim MA & Warda M: Molecular characterization of alpha subunit 1 of sodium pump (ATP1A1) gene in Camelusdromedarius: its differential tissue expression potentially interprets the role in osmoregulation. Molecular Biology Reports 2022; 49(5): 3849-3861.
- Lambe MO & Bewaji CO: Moringa oleifera leaf-based diet combats malnutrition in rats. Biokemistri 2022; 33(2).
- Liu M, Yang H & Mao Y: Magnesium and liver disease. Annals of Translational Medicine 2019; 7(20).
- Lopez-Rodriguez NA, Gaytán-Martínez M, de la Luz Reyes-Vega M and Loarca-Pina G: Glucosinolates and isothiocyanates from Moringa oleifera: Chemical and biological approaches. Plant Foods Hum Nutr 2020; 75: 447–457.
- Meireles D, Gomes J, Lopes, L. Hinzmann M and Machado J: A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: integrative approach on conventional and traditional Asian medicine. Adv Tradit Med 2020; 20: 495–515.
- Mushtaq BS, Hussain MB, Omer R, Toor HA, Waheed M, Shariati MA, Sergey P and Heydari M: Moringa oleifera in malnutrition: A comprehensive review. Curr Drug Discov Technol 2021; 18(2): 235-243.
- Ngaha EO, Akanji MA & Madusolumuo MA: Studies on correlations between chloroquine-induced tissue damage and serum enzyme changes in the rat. Experientia 1989; 45: 143-146.
- Ogbu SI & Okechukwu EI: The effect of storage temperature prior to separation on plasma and serum potassium. J Med Lab Sci 2001; 10: 1-4.
- Ogunniyi KAB: A Study of Protein Energy Malnutrition (PEM) Among Under-Five Children Okuku, Odootin Local Government Area of Osun State, Nigeria. Adeleke University Journal of Science 2022; 1(1): 258-266.
- Olayinka LM & Clement B: Protective role of Moringa oleifera leaf-based diet on protein-energy malnutrition induced skeletal muscle degeneration. Int J Sci Rep 2017; 3(2): 54-62.
- Radosinska J & Vrbjar N: Erythrocyte deformability and Na, K-ATPase activity in various pathophysiological situations and their protection by selected nutritional antioxidants in humans. International Journal of Molecular Sciences 2021; 22(21): 11924.
- Raphael JE, Atanu FO, Mohammed SS & Abdulrahman, SI: Anti-anaemic effects of Moringa oleifera seeds (Lam) in protein energy malnourished rats. GSC Biological and Pharmaceutical Sciences 2020; 13(1): 197-202.
- Reitman S and Frankel S: Colometric methods for aspartate and alanine aminotransferase. American Journal of Clinical Pathology 1957; 28: 55-60.
- Saini RK, Sivanesan I and Keum YS: Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. Biotech 2016; 6: 203.
- Saucedo-Pompa S, Torres-Castillo JA, Castro-López C, Rojas R, Sánchez-Alejo EJ, Ngangyo-Heya M and Martínez-Ávila GCG: Moringa plants: Bioactive compounds and promising applications in food products. Food Res Int 2018; 111: 438-450.
- Singh VP, Arulanantham A, Parisipogula V, Arulanantham S and Biswas A: Moringa olifera: Nutrient dense food source and world’s most useful plant to ensure nutritional security, good health and eradication of malnutrition. Euro J Nutr Food Saf 2018; 8(4): 204-14.
- Sreeja M, Jayasri P, Keerthi N, Yeshashwini J & Praveen J: Moringa oleifera: A review on nutritive importance and its potential use as nutraceutical plant. Journal of Medicinal Plants 2021; 9(2): 15-7.
- Sujatha BK & Patel P: Moringa oleifera nature’s gold. Imperial Journal of Interdisciplinary Research 2017; 3(5): 1175-1179.
- Zhu Y, Yin Q and Yang Y: Comprehensive investigation of Moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules 2020; 25(3): 676.
How to cite this article:
David NC, Victor OM, Godwin MA, Lucky E, Chiamaka FN, Mary O, Oyewole BO, Theresa OO, Helen EO, Rebecca OG and Toheeb M: Effects of defatted Moringa oleifera seed on skeletal muscle of protein energy malnourished rats. Int J Pharmacognosy 2023; 10(12): 571-77. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.10(12).571-77.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
571-577
517 KB
663
English
IJP
N. Charles David *, Okoliko Micheal Victor, Mathias Akoji Godwin, Ekwoba Lucky, F. Nwobodo Chiamaka, Ozonkwo Mary, B. Olorunfemi Oyewole, O. Okeme Theresa , E. Omeje Helen, O. Gift Rebecca and Muritadoh Toheeb
Department of Biochemistry, Olusegun Agagu University of Science and Technology, Okitipupa, Nigeria.
charlesdavidnw@gmail.com
27 October 2023
19 December 2023
27 December 2023
10.13040/IJPSR.0975-8232.IJP.10(12).571-77
31 December 2023