EFFECT OF SOFT DRINKS ON IN-VITRO KIDNEY STONE (CALCIUM OXALATE MONOHYDRATE)
HTML Full TextEFFECT OF SOFT DRINKS ON IN-VITRO KIDNEY STONE (CALCIUM OXALATE MONOHYDRATE)
Ayesha Tajammul 1, Nurul Kabir 1, Maryam Ahmed * 2 and Ali Sufyan 3
Dr. Panjwani Center for Molecular Medicine and Drug Research 1, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi, Pakistan.
Department of Pharmacognosy, Faculty of Pharmacy 2, Federal Urdu University of Arts, Science and Technology, Karachi, Pakistan.
Department of Microbiology 3, University of Karachi, Karachi, Pakistan.
ABSTRACT: Kidney stone is a painful disease which is a major problem in all regions of Pakistan as this country is located in the so-called stone belt. Drastic changes in dietary habits including the use of soft drinks are increasing the incidence of kidney stone disease. To investigate this phenomenon, calcium oxalate monohydrate crystals were induced in artificial urine by mixing calcium chloride and sodium oxalate in-vitro and different types of soft drinks were analyzed for activity against calcium oxalate monohydrate type’s kidney stone. Most of them showed effects on calcium oxalate monohydrate to calcium oxalate dihydrate crystals under DIC microscopy. This study showed that slices had the most promising effect on calcium oxalate monohydrate crystals than Mirinda and Mountain dew. 7up and Pepsi did not show a change in morphology of the crystals of calcium oxalate monohydrate.
| Keywords: |
In-vitro, Kidney stone, Calcium oxalate monohydrate, Calcium oxalate dihydrate, Soft drink
INTRODUCTION: Kidney stone disease is as old as humans and has been found in the tombs of Egyptian mummies dating back to 4000 BC 1. In the past, it was considered a disease with terrible upshots which frequently lead to death. The epidemiology of kidney stone in a given population varies according to the geographical area, climate and season. Pakistan is situated in the Afro-Asian stone belt 2. Most of the cases of kidney stones have been reported in the summer rather than the winter in a given population 3. People of all ages are susceptible to kidney stone disease.
Prevalence of kidney stone varies according to the sex, and 20% of the females and 4% of the males are affected by this disease 4. The changes in dietary habits, such as having fast food rather than traditional food, increase the chance of stone formation, as well as abnormal intake of fast foods, soft drinks, sodium, and oxalate-containing vegetables and fruits take part in the progression of kidney stone disease 5.
Calcium oxalate crystallization is a very complex and fundamental step in the formation of kidney stones. This process includes; urinary super-saturation, crystal nucleation, crystal growth, aggregation, and crystal-cell interaction. Many researchers have proposed in-vitro assay to evaluate the activity of different plant’s extracts along with their isolated or synthesized compounds against kidney stone 6. This study is based on in- vitro kidney stones formation in artificial urine containing pathological calcium oxalate monohydrate crystals and their modulation or morphological change into non-pathological calcium oxalate dihydrate crystals 7 by a variety of soft drinks acting against these kidney stones in- vitro. This activity can be determined by spectro-photometry 8 and microscopy 9.
MATERIALS AND METHODS:
Soft Drinks: Different varieties of soft drinks were purchased from the departmental store of Karachi which included Slice, Mirinda, Mountain dew, 7up, and Pepsi. Approximately 5, 10, 15 and 20 µL of each drink were used for this study to evaluate the activity against calcium oxalate monohydrate kidney stone.
Spectrophotometric Analysis: After making artificial urine in a 96-well flat-bottomed plate by mixing 100 µL calcium chloride (4 mmol/L), 100 µL sodium oxalate (0.5 mmol/L), 200 mmol/L sodium chloride and 10 mmol/L sodium acetate, calcium oxalate stones were induced in-vitro 8. This artificial urine was adjusted to 5.7 pH. The optical density was measured after each five minutes interval throughout 80 min at 620 nm at 37 °C using a Sunrise Microplate Reader (Tecan, Austria GmbH) with and without the addition of different soft drinks during crystallization in artificial urine.
Microscopic Studies: After the spectrophotometric determination, the effects of soft drinks were observed on calcium oxalate monohydrate crystals under the Differential Interference Contrast (DIC) microscope. Using polarized light, crystals were distinguished on the bases of their morphology. A Nikon Eclipse TE2000-E microscope (Nikon, Japan) and Nikon DXM 1200C camera were used to obtaining the images. The images were analyzed by using NIS-Elements image analysis software AR 3.2 (Nikon, Japan).
Statistics: Statistical calculations were performed by Microsoft Excel 2003 and p-value of 0.001-0.05 was measured to be significant in t-tests.
RESULTS AND DISCUSSION: To determine the activity of soft drinks against kidney stone in-vitro, calcium oxalate monohydrate crystallization were analyzed by spectrophotometry and observed under DIC microscope and results are described in Table 1.
TABLE 1: SPECTROPHOTOMETERIC AND MICROSCOPIC DETERMINATION OF EFFECT OF SOFT DRINKS AGAINST CALCIUM OXALATE CRYSTALLIZATION
| S. no. | Name of soft drink | 5 µl/mL | 10 µl/mL | 15 µl/mL | 20 µl/mL |
| 1 | Slice | + | ++ | +++ | ++++ |
| 2 | Mirinda | + | + | + | + |
| 3 | Pepsi | - | - | - | - |
| 4 | Mountain Dew | + | + | + | + |
| 5 | 7up | - | - | - | + |
Spectrophotometric Analysis: Slice showed excellent while mirinda and mountain dew showed moderate activity against calcium oxalate monohydrate crystals in-vitro. Pepsi and 7up did not show noticeable activity against calcium oxalate monohydrate kidney stone.
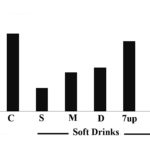
FIG. 1: SPECTROPHOTOMETRIC ANALYSIS OF SOFT DRINKS AGAINST CALCIUM OXALATE CRYSTALLIZATION IN ARTIFICIAL URINE. In-vitro, calcium oxalate monohydrate stone was induced and optical density was measured at 620 nm throughout 80 min. Slice decreased the optical density more significantly than other soft drinks. C, S, M, D, and P represent the control group, Slice, Mirinda, dew and Pepsi respectively.
Spectrophotometric analysis Fig. 1 showed that slice has considerable effect against in-vitro calcium oxalate monohydrate crystals than Mirinda and Mountain dew by decreasing the optical density at 620 nm as compared with control. No change was observed in the optical density of 7up and Pepsi when reading against a control group containing calcium oxalate monohydrate crystals.
Microscopic Determination:
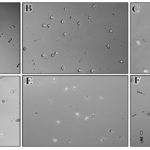
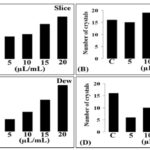
Slice: Elongated rod-shaped calcium oxalate monohydrate (COM) crystals were observed under DIC microscope Fig. 2A in 200x magnification with 15 different fields. Slice juice with a dosage of 5, 10, 15 and 20 µL/mL were used to evaluate the activity against COM in-vitro kidney stones. Pyramidal shaped calcium oxalate dihydrate (COD) stone was observed under DIC microscope when treated with a slice Fig. 2B. Approximately 16 ± 3.3 COM crystals were observed under DIC microscope Fig. 3A and their size was 150 ± 1 µm2 under control conditions Fig. 4A. The numbers of the stones Fig. 3A were found 18 ± 1.1, 20 ± 4.2, 28 ± 7.5 and 34 ± 6.0 with 5, 10, 15 and 20 µL/mL of slice juice respectively. The sizes of the stones Fig. 4A were 286.1 ± 1.3, 252 ± 3.6, 199.3 ± 4.8 and 172 ± 9.1 µm2 with 5, 10, 15 and 20 µL/mL of slice respectively. Slice showed its effect as dose-dependent increased in stone number and decrease in stone size which suggested significant activity against in-vitro COM kidney stone.
FIG. 2: IN-VITRO EFFECT OF SOFT DRINKS ON KIDNEY STONE CRYSTAL’S MORPHOLOGY WAS OBSERVED BY DIC MICROSCOPY. In-vitro assay against kidney stone was observed under DIC microscopy at a magnification of 200X. Under control conditions (A) only rod-shaped calcium oxalate monohydrate crystals were observed. At constant dose (15 ml/mL) of different soft drinks, pyramidal-shaped COD crystals were observed with a slice (B) whereas hexagonal shaped crystals were observed with mirinda (C) and mountain dew (D). No or very little effects were observed with pepsi (E) and 7up (F) respectively. Scale bar is 50 mm
Mirinda and Mountain Dew: Spectrophotometric assay suggested the moderate activity of the mirinda and mountain dew against the formation of the kidney stone Fig. 1 as compared with control. When COM crystals were treated with mirinda and mountain dew hexagonal shaped calcium oxalate crystals were observed in the similar dosage of 5, 10, 15 and 20µL/mL of mirinda Fig. 2C and mountain dew Fig. 2D under DIC microscope. These types of crystals are also reported as in inert form to bind with damaged kidney cells 10.
The number of the crystals in 200x magnification field were 16 ± 3.3, 15 ± 1.0, 19 ± 11.6, 17 ± 13.1, 18 ± 7.3 with control, 5, 10, 15 and 20 µL/mL of mirinda Fig. 3B respectively. Whereas, the number of the crystals were 16 ± 3.3, 5 ± 7.1, 8 ± 15.1, 13 ± 5.1, 19 ± 7.3 with control, 5, 10, 15 and 20 µL/mL of mountain dew Fig. 3C respectively. The sizes of the stones were 150 ± 1, 299.9 ± 17.0, 352.1 ± 11.0, 328.9 ± 14.8, and 179.9 ± 10.7 µm2 with control, 5, 10, 15 and 20 µL/mL of mirinda Fig. 4B respectively.
FIG. 3: IN-VITRO EFFECT OF DIFFERENT DOSAGE OF SOFT DRINKS ON COUNTING OF CALCIUM OXALATE STONES. Soft drinks affected on a number of stone by different manners and showed activity against calcium oxalate crystals. Slice (A), Mirinda (B), Mew (C) and 7up (D) showed different effects on crystals number.
FIG. 4: IN-VITRO EFFECT OF DIFFERENT DOSAGE OF SOFT DRINKS ON SIZE OF CALCIUM OXALATE STONES. Soft drinks affected on the size of stone by different manners and showed activity against calcium oxalate monohydrate crystals. Slice (A), Mirinda (B), Dew (C) and 7up (D) showed different effects on crystal size.
Whereas the sizes of the stones were 150 ± 1, 409 ± 10.0, 385 ± 9.0, 356.7 ± 8.2 and 304 ± 13.3 µm2 with control, 5, 10, 15 and 20 µL/mL of mountain dew Fig. 4C respectively. These changes in morphology of COM crystals may be due to the chelation of calcium by citrate present in both Mirinda and Mountain dew. These changes were not similar as observed in the slice, therefore, showed moderate activity against in-vitro calcium oxalate kidney stones.
7up and Pepsi: 7up & pepsi did not show activity against in-vitro calcium oxalate monohydrate kidney stones. Under DIC microscopic examination with 200x magnification, the shape of stones was observed with the rod to hexagonal with increasing in size and decreasing in a number of the crystals after treatment with 7up Fig. 2E, but the effect was opposite to Mirinda and Mountain dew with similar dosage. The numbers of the stones Fig. 3D were found 16 ± 3.3, 16 ± 5.1, 10 ± 14.2, 6 ± 17.5 and 5 ± 8.0 with control, 5, 10, 15 and 20 µL/mL of 7up respectively. The sizes of the stones Fig. 4D were 150 ± 1, 210.1 ± 5.3, 245 ± 12, 305.2 ± 12.1 and 384 ± 11.1 µm2 with control, 5, 10, 15 and 20 µL/mL of 7up respectively. DIC microscopic examination confirmed the presence of COM crystals even after treatment of control crystals to Pepsi Fig. 2F which did not show any conformational change in crystals morphology 11-13.
CONCLUSION: In this study, we suggested that soft drinks effect on calcium oxalate monohydrate kidney stone. Effect of soft drinks including Slice, Mirinda, Mountain dew, 7up and Pepsi was evaluated to determine in-vitro activity against calcium oxalate kidney stones by using spectro-photometry and DIC microscope. Slice was observed with significant and Mirinda as well as mountain dew showed moderate activity against kidney stone whereas 7up and Pepsi did not show any effect against in-vitro calcium oxalate monohydrate crystals. Slice, Mirinda and Mountain dew greatly affected the stone formation and reduced the stone size but increased the stone number.
These changes in crystals morphology are beneficial because COM crystals are associated to bind with cells of the damaged kidney to produce kidney stone disease. Physiological COD crystals are harmless because they are less able to adherence to damaged kidney cells and excreted out from the body through the urination.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Riches E: The history of lithotomy and lithotrity. Ann R Coll Surg Engl 1968; 43: 185-199
- López M and Hoppe B: History, epidemiology and regional diversities of urolithiasis. Pedi Nephrol 2010; 25: 49-59
- Sternberg K, Greenfield SP, Williot P and Wan J: Pediatric stone disease: an evolving experience. J Urol 2005; 174: 1711-1714.
- Robertson WG: Renal stones in the tropics. Semin Nephrol 2003; 23: 77-87.
- Curhan GC: Dietary calcium, dietary protein, and kidney stone formation. Miner Electrolyte Metab 1997; 23: 261-264
- Atmani F: Medical management of urolithiasis, what opportunity for phytotherapy? Front Biosci 2003; 8, s507-514.
- Chen Z, Wang C, Zhou H, Sang L and Li X: Modulation of calcium oxalate crystallization by commonly consumed green tea. Cryst. Eng. Comm 2009; 12: 845-852.
- Kulaksizoglu S, Sofikerim M and Cevik C: In-vitro effect of lemon and orange juices on calcium oxalate crystallization. Int Urol Nephrol 2008; 40: 589-594
- Barros ME, Schor N and Boim MA: Effects of an aqueous extract from Phyllanthus niruri on calcium oxalate crystallization in-vitro. Urol Res 2003; 30: 374-379
- Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y and Ohri K: Preventive effects of green tea on the renal stone formation and the role of oxidative stress in nephrolithiasis. The J of Urol 2005; 173: 271-275.
- Lieske JC and Toback FG: Regulation of renal epithelial cell endocytosis of calcium oxalate monohydrate crystals. Am J Physiol 1993; 264: F800-807.
- Wesson JA, Worcester EM, Wiessner JH, Mandel NS and Kleinman JG: Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int 1998; 53: 952-957.
- Chen Z, Wang C, Zhou H, Sang L and Li X: Modulation of calcium oxalate crystallization by commonly consumed green tea. Cryst Eng Comm 2009; 12: 845-852.
How to cite this article:
Tajammul A, Kabir N, Ahmed M and Sufyan A: Effect of soft drinks on in-vitro kidney stone (Calcium oxalate monohydrate). Int J Pharmacognosy 2014; 1(6): 399-03. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(6).399-03.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
8
399-403
694
2362
English
IJP
A. Tajammul, N. Kabir, M. Ahmed * and A. Sufyan
Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi, Pakistan
maryamahd@yahoo.com
12 April 2014
26 May 2014
28 May 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(6).399-03
01 June 2014