DEVELOPMENT OF QUALITY PARAMETERS AND DETERMINATION OF GLYCYRRHETINIC ACID AND PIPERINE CONTENT IN YASTIMADHUVATI
HTML Full TextDEVELOPMENT OF QUALITY PARAMETERS AND DETERMINATION OF GLYCYRRHETINIC ACID AND PIPERINE CONTENT IN YASTIMADHUVATI
Vipul Chaudhary, Mamta Shah and Karuna Modi *
Department of Pharmacognosy and Phytochemistry, L. M. College of Pharmacy, Ahmedabad, Gujarat, India.
ABSTRACT: `Yastimadhuvati’ is a popular Ayurvedic formulation prescribed in cough, cold, respiratory diseases and chronic fever. The proposed study is aimed at developing physico-chemical parameters and estimating the contents of glycyrrhetinic acid and piperine in the formulation. Pills were prepared according to the Vaidyaka chikitsasar. The pills were subjected to pharmacognostical and phytochemical screening and evaluated for the standards visually, weight variation, friability and hardness tests. Further glycyrrhetinic acid was quantified by colorimetric method. Also a simultaneous HPTLC method was developed for quantification of glycyrrhetinic acid and piperine. The pills showed microscopical features like sclereids, crystal fibres, vittae, epidermis with subepidermis and oil glands. The acid insoluble ash was found to be 0.5 ± 0.61, Water soluble extractive value was higher than the alcohol soluble extractive value. The pills contained higher amounts of phenolics. The content of glycyrrhetinic acid was found to be almost same (0.4% w/w) when estimated by colorimetric and HPTLC methods. Piperine content was found to be 3.5%w/w using the same HPTLC method. The quality parameters developed for the Yastimadhuvati would serve as useful tool for gauging the quality and also the simultaneous HPTLC method developed for the estimation of glycyrrhetinic acid and piperine is simple and reproducible.
Keywords: Colorimetry, Glycyrrhetinic acid, HPTLC, Piperine, Yastimadhuvati
INTRODUCTION: Medicines prepared in the form of pills or tablets are known as `Vati’ or `Gutika’ which contains one or more drugs obtained from plants, animals or mineral origin. Yastimadhuvati, a well known Ayurvedic formulation, is commonly used in cough and respiratory diseases 1. The major ingredients of the vati (pills) include glycyrrhiza, black pepper, cardamom, beheda, fennel, black catechu, clove and peppermint.
A standard formulation was preparedin laboratory as mentioned in Vaidyakachikitsasar and evaluated for quality parameters and content of glycyrrhetinic acid by both colorimetric 2 and HPTLC methods and piperine content by HPTLC method.
MATERIALS AND METHODS:
Plant Materials and Preparation of Formulation: Crude drugs for formulation were collected from local market of Ahmedabad and were identified by morphological characters. The raw materials were powdered separately to 60 mesh size and Yastimadhuvati was prepared according to the formulation of Vaidyakachikitsasar Table 1. Pills of 5mm were prepared mixing the powdered material using distilled water and dried at room temperature till they attained constant weight.
TABLE 1: YASTIMADHUVATI FORMULATION
| Sr. no. | Common Name | Latin Name | Part used | Quantity (Tola) |
| 1 | Baheda | Terminalia belerica Linn. | Fruit | 4 |
| 2 | Variyali | Foeniculum vulgare Miller. | Fruit | 4 |
| 3 | Elaichi | Elettaria cardamom Maton. | Seed | 4 |
| 4 | Jethimadha | Glycyrrhiza glabra Linn. | Root or stolon | 4 |
| 5 | Jethimadha no shiro | Glycyrrhiza glabra Linn. | Aqueous extract | 16 |
| 6 | Kali mirch | Piper nigrum Linn. | Fruit | 4 |
| 7 | Laving | Eugenia caryophyallus Thunb. | Flower bud | 4 |
| 8 | Katha | Acacia catechu Wild. | Heart wood | 4 |
| 9 | Pippermint | Various species of Mentha | - | 1 |
Evaluation of Parameters of Yastimadhuvati: Yastimadhuvati was subjected to evaluation for weight variation, friability, hardness and disintegrationtests 3.
Pharmacognostical and Phytochemical Analysis: The pills were powdered and studied for the microscopical characters, ash values, extractive values 4 and presence of various secondary metabolites 5-8. The content of volatile oil 4, alkaloids 9, flavanoids 10, phenolics 10 and tannins 11 were also estimated.
Estimation of Glycyrrhetinic Acid by Colorimetric Method 2: The content of glycyrrhetinic acid in the pills was estimated by the method of Srivastava et al. The powdered vati after suspending in dioxane was subjected to hydrolysis using H2SO4. The hydrosylate was neutralized and shaken with CHCl3. The CHCl3 extract after drying was treated with H2SO4 followed by alcoholic solution of vanillin. The mixture was kept in boiling water bath for five minutes and optical density was measured at 545 nm (Schimadzu UV 1700, Japan).
Estimation of Glycyrrhetinic Acid and Piperine by HPTLC: A simultaneous HPTLC method was developed to quantify glycyrrhetinic acid and piperine in yastimadhuvati. Reagents like 1,4-dioxane, toluene, ethylacetate, methanol and ammonia used were of chromatography grade. Reference standard of glycyrrhetinic acid and piperine were purchased from Sigma Aldrich, India. The HPTLC was done on precoated TLC plates of silica gel 60 F254 (Merck)as stationary phase and 1,4-Dioxane: toluene: ethyl acetate: methanol: ammonia (1.5:2:1: 1:0.3) as mobile phase using Camag Linomat V equipped with Camag TLC Scanner and Win CATS integration software(version 1.4.3.6336).
Calibration curves of reference standards were prepared using stock solution (1mg/ml) of piperine and glycyrrhetinic acid in methanol. A fixed volume of standard solution (1, 2, 3, 4, 5 μl) was spotted. Calibration curves of peak area vs. concentration of standard were plotted. The test solution was prepared extracting 10 g of yastimadhuvati powder with 50ml distilled water exhaustively. It was hydrolyzed with 10 ml 2N H2SO4, neutralized and extracted with 50 ml of chloroform twice. The residue after removing CHCl3 was weighed and 5mg/ml solution was prepared and; 10 and 20μl of this was spotted. The plates were visualized and scanned at 254 nm. Derivatization was done using anisaldehyde sulphuric acid for glycyrrhetinic acid and dragendorff’s reagent for piperine.
RESULT AND DISCUSSION: Yastimadhuvati was round shaped and dark brown to black coloured with aromatic odour and astringent taste. Powdered yastimadhuvati can be characterized by presence of crystal fibres, vittae, epidermis with subepidermis, group of stone cells, sclereides and oil glands Fig. 1.
Total ash, water-soluble ash and acid insoluble ash values were found to be 7.8 ± 0.76, 3.80 ± 0.27 and 0.5±0.61%w/w, respectively indicating very less extent of earthy matter in the formulation. Water soluble extractive was found to be appreciably higher than alcohol soluble extractive values.
The value of loss on drying and volatile oil content were 26.41±0.40 % w/w and 10.6±0.11% v/w respectively revealing significance for aromatic drugs used in formulation Table 2. Yastimadhuvati complied with IP limits for weight variation test, friability test, hardness and disintegration tests Table 3.
Phytochemical screening revealed the presence of saponins, alkaloids, flavanoids, sterol and triterpenoids, phenolics, tannins, and carbohydrates. Among the phytoconstituents estimated, total phenolic content was the highest Table 4.
FIG. 1: MICROSCOPICAL CHARACTERS OF YASTIMADHUVATI
TABLE 2: PHYSICO-CHEMICAL PARAMETERS OF YASTIMADHUVATI
| Quality parameter | Percentage |
| Water soluble extractive | 35.3 ± 0.98 % w/w |
| Alcohol soluble extractive | 8.6 ± 0.41 % w/w |
| Loss on drying | 26.41 ± 0.40 % w/w |
| Volatile oil content | 10.6 ± 0.11 % v/w |
Standard deviation (±SD), Number of reading (n) = 3.
TABLE 3: STANDARD PARAMETERS OF YASTIMADHUVATI
| Parameters | Value |
| Weight variation test
No. of vat is showing deviation from average weight by more than 7.5% No. of vat is showing average weight ± 7. 5% |
15
5 |
| Disintegration test (min) | 34.5 |
| Friability (%) | 0.1 |
| Hardness test (kg/cm2) | 6.8 |
TABLE 4: PHYSICO-CHEMICAL PARAMETERS OF YASTIMADHUVATI
| Phytoconstituents | %w/w |
| Alkaloids | 0.542 ± 0.00 |
| Phenolics | 14.1 ± 0.26 |
| Flavanoids | 1.324 ± 0.42 |
| Tannins | 1.23 ± 0.02 |
Standard deviation (±SD), Number of reading (n) = 3.
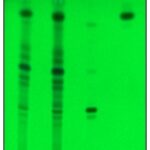
Glycyrrhetinic acid was estimated by colorimetric assay method 0.42±0.12% w/w in yastimadhuvati. Simultaneous HPTLC method for quantification of glycyrrhetinic and piperine was found to be sensitive, reproducible and suitable for routine analysis. A simple sample preparation method was generated, which was economic for the purpose of quality control. Co-chromatography with reference standards glycyrrhetinic acid and piperine revealed resolution of the same in yastimadhuvati, at Rf 0.31 and 0.82 Fig. 2.
The content of glycyrrhetinic acid and piperine indicated 0.40±0.005% w/w and 3.5±0.01% w/w respectively. Also glycyrrhetinic acid content was found to be almost similar when estimated by colorimetric method. The results of the present study would serve as a valuable gauge for evaluation of quality of yastimadhuvati.
FIG. 2: TLC OF YASTIMADHUVATI
CONCLUSION: The present study successfully established quality control parameters for Yastimadhuvati and developed reliable methods for quantification of its key phytoconstituents, glycyrrhetinic acid and piperine. Pharmacognostical, phytochemical, and physicochemical evaluations confirmed the presence of characteristic features and revealed significant phenolic content, thereby validating the formulation’s traditional use. The content of glycyrrhetinic acid determined by both colorimetric (0.42% w/w) and HPTLC (0.40% w/w) methods showed close agreement, while piperine was quantified as 3.5% w/w using HPTLC. The developed HPTLC method proved simple, sensitive, reproducible, and cost-effective, making it suitable for routine quality assessment. These findings provide a scientific basis for standardization of Yastimadhuvati, ensuring its quality, safety, and efficacy. The study thus contributes to strengthening quality assurance practices in Ayurvedic formulations and may serve as a reference for future pharmacopoeial standards.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Thakkur GK: Vaidyakachikitsasar. Sindh Ayurvedic Pharmacy, First Edition 1936; 1.
- Srivastava VK, Mukerjee SK and Maheshwari ML: Estimation of glycyrrhitinic acid in glycyrrhiza roots. Indian Drugs 1977; 14(4): 80-82.
- Anonymous: Indian Pharmacopoeia. Ministry of Health and Family Welfare 2007; 1.
- Anonymous: Ayurvedic Pharmacopoeia of India. Ministry of Health and Family welfare, First Edition 1999; 2.
- Sim SK: Medicinal Plant Alkaloids. University of Toronto Press 1969.
- Geissman A: Modern Methods of Plant Analysis. Springer Verlag 1955; 3: 471.
- Evans WC and Evans D: Pharmacognosy. WB Saunders Company Ltd., Fifteenth Edition 2002.
- Robinson T: The organic constituents of higher plants, their chemistry and interrelationships. Burgers Publishing Company 1964.
- Sreevidya N and Mehrotra S: Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. Journal of AOAC International 2003; 86(6): 1124-1127.
- Kalola J and Shah M: Free radical scavenging activity of Inula cappa. Ars Pharmaceutica 2006; 47(4): 387-388.
- Anonymous: State Pharmacopoeia of the Union of Soviet Socialist Republics IX. Ministry of health of USSR, Ninth Edition 1961.
How to cite this article:
Chaudhary V, Shah M and Modi K: Development of quality parameters and determination of glycyrrhetinic acid and piperine content in yastimadhuvati. Int J Pharmacognosy 2025; 12(9): 762-65. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.12(9).762-65.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
9
762-765
828 KB
155
English
IJP
Vipul Chaudhary, Mamta Shah and Karuna Modi *
Department of Pharmacognosy and Phytochemistry, L. M. College of Pharmacy, Ahmedabad, Gujarat, India.
karuna.modi@lmcp.ac.in
12 September 2025
28 September 2025
29 September 2025
10.13040/IJPSR.0975-8232.IJP.12(9).762-65
30 September 2025