DETERMINATION OF BIOACTIVE COMPOUNDS OF EQUISETUM ARVENSE BY GAS CHROMATOGRAPHY-MASS SPECTROMETRY METHOD
HTML Full TextDETERMINATION OF BIOACTIVE COMPOUNDS OF EQUISETUM ARVENSE BY GAS CHROMATOGRAPHY-MASS SPECTROMETRY METHOD
Semih Yilmaz 1, Sernur Ekinci 1 and Bilal Yilmaz * 2
Mehmetcik Middle School 1, Palandoken, 25070, Erzurum, Turkey.
Department of Analytical Chemistry 2, Faculty of Pharmacy, Ataturk University, 25240, Erzurum, Turkey.
ABSTRACT: Equisetum arvense has a great medicinal value for its wound and burn healing properties. Traditionally, the plant is used by local people and Ayurvedic physicians mainly for its burn healing properties. The methanolic extract of Equisetum arvense was obtained by extraction. The present study focuses on the analysis of the methanol extract of Equisetum arvense by Gas Chromatography-Mass Spectrometry. The phytocomponents of the methanol extract of Equisetum arvense were investigated by using Gas Chromatography-Mass spectrometry, while the mass spectra of the compounds found in the extract were matched with the National Institute of Standards and Technology Library. The study revealed the presence of six phyto-components.
| Keywords: |
Equisetum arvense, Bioactive compounds, Gas Chromatography-Mass Spectrometry
INTRODUCTION: Herbal medicine, as a major part of traditional medicine, has been used in medical practice since antiquity and is a common element of ayurvedic, homeopathic, and naturopathic medicine. World health organization notes that 74% of the plant-derived medicines are used in modern medicine, in a way that their modern application directly correlates with their traditional use as herbal medicines by native cultures 1, 2.
Equisetum arvense Fig. 1 (Family: Equisetaceae) commonly known as the Field Horsetail or Common Horsetail, is a bushy perennial herb native to the northern hemisphere. It is a member of a very primitive family of plants.
In spring a spore-bearing stem, resembling a thin asparagus shoot, rises 15-20 cm; once shed, a pale green bush replaces this with erect, hollow jointed stems with longitudinal furrows, and with sharply toothed sheaths covering each joint; from the sheaths of the central stem arise whorls of fine branches, each giving off finer whorls, the whole sometimes extending up to 60 cm in height 3, 4.
FIG. 1: EQUISETUM ARVENSE PLANT
Active compounds of the plant include minerals like silicic acids and silicates, potassium, sulphur, manganese, magnesium; flavonoids: quercetin glycosides; phenolic acids, alkaloids, equisetonin, phytosterols: cholesterol, isofucosterol, campesterol; tannins 5, 6. Horsetail possesses diuretic properties, which are believed to be due to equisetonin and flavone glycosides 7. Horsetail herb extract helps body retain calcium more efficiently due to a silica compound and can even help repair bones and cartilage. This is certainly essential for managing joint degeneration conditions or hard to heal bone fractures. Osteoporosis is one among many diseases that horsetail extract benefits 8. Horsetail is known for its anti-inflammatory, antinociceptive 9, antioxidant and antiproliferative 10, antimicrobial 11-13, hepatoprotective 14, antidiabetic 15, coagulant and astringent activity 16.
A knowledge of the chemical constituents of horsetails is desirable not only for the discovery of therapeutic agents but also because such information may be of great value in disclosing new sources of economic phytocompounds for the synthesis of complex chemical substances and for discovering the actual significance of folkloric remedies. Hence a thorough validation of the herbal drugs has emerged as a new branch of science emphasizing and prioritizing the standardization of the natural drugs and products because several of the phytochemical have a complementary and overlapping mechanism of action. Mass spectrometry, coupled with chromatographic separations such as Gas chromatography (GC-MS) is normally used for direct analysis of components existing in traditional medicines and medicinal plants. With this background, the present study was aimed to identify six phytocomponents of Equisetum arvense using GC-MS analysis.
MATERIALS AND METHODS:
Chemicals and Reagents:
Apparatus and Analytical Conditions: Chromatographic analysis was carried out on an Agilent 6890N gas chromatography system equipped with 5973 series mass selective detector, 7673 series autosampler and chemstation (Agilent Technologies, Palo Alto, CA). HP-5 MS column with 0.25 μm film thickness (30 m × 0.25 mm I.D., USA) was used for separation. The splitless injection was used, and the carrier gas was helium at a flow rate of 1 mL min-1. The injector and detector temperatures were 250 ºC. The MS detector parameters were transferred line temperature 290 ºC, solvent delay 3 min and electron energy 70 eV. The MS was run in scan mode (m/z 40-500) for qualitative analysis.
Extraction Procedure: Equisetum arvense plant was collected in June 2013 from Uzundere, Erzurum. 1 g the powdered Equisetum arvense plant was soaked in methanol for 12 h. The extracts were then filtered through Whatmann filter No. 42. The filtrate was then concentrated by bubbling nitrogen gas into the solution. The extract contained both polar and non-polar phytocomponents in the plant material. 1 μl of this solution was employed for GC-MS analysis.
Identification of Components: Identification was based on the molecular structure, molecular mass, and calculated fragments. Interpretation on mass spectrum GC-MS was conducted using the database on National Institute Standard and Technology having more than 62,000 patterns. The name, molecular weight, and structure of the components of the test materials were ascertained. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. The spectrum of the unknown component was compared with the spectrum of the component stored in the National Institute of Standards and Technology Library Version (2005), Software, Turbomass 5.2.
RESULTS AND DISCUSSIONS:
Method Development and Optimization: The method development for the assay of phytocomponents was based on their chemical properties. In this study, the capillary column coated with 5% phenyl, 95% dimethylpolysiloxane is a good choice for separation of these analytes since they elute as symmetrical peaks at a wide range of concentrations. Different temperature programs were investigated for GC oven. The end of this investigation, the best temperature program was selected for good separation. The temperature programs of the GC oven were as follows: initial temperature 80 ºC, held for 1 min, increased to 280 ºC at 12 ºC min-1 held for 1 min, and finally to 300 ºC at 5 ºC min-1 with a final hold of 1 min. The splitless injection mode was chosen. Additionally, preliminary precision and linearity studies performed during the development of the method showed that the 1 µL injection volume was reproducible and the peak response was significant at the analytical concentration chosen.
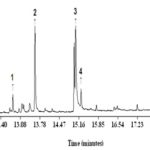
GC-MS Analysis: The more precise information in qualitative analysis can be obtained by gas chromatography coupled with mass spectrometry. For quantitative determination, gas chromato-graphy with flame ionization detector (GC-FID) and GC-MS are preferred. GC-MS is one of the best techniques to identify the constituents of volatile matter, long chain, branched chain hydrocarbons, alcohols, acids, esters etc. The GC-MS analysis of Equisetum arvense extract revealed the presence of six compounds (phytochemical constituents) that could contribute to the medicinal quality of the plant. The identification of the phytochemical compounds was confirmed based on the peak area, retention time and molecular formula. The major phytochemical constituent’s present in the methanolic extract of Equisetum arvense is presented as compound chromatogram is in Fig. 2.
FIG. 2: GC-MS CHROMATOGRAM OF METHANOLIC EXTRACT OF EQUISETUM ARVENSE
The first compound identified with less retention time (12.8 min) was 3, 7, 11, 15-tetramethyl-2-hexadecene-1-ol, whereas gibberellic acid was the last compound which took longest retention time (20.7 min) to identify. The phytochemicals identified through GC-MS analysis showed many biological activities relevant to this study are listed in Table 1.
2-Hexadecene-1-ol and 3, 7, 11, 15-tetramethyl are a fragrance ingredient used in many fragrance compounds. It may be found in fragrances used in decorative cosmetics, fine fragrances, shampoos, toilet soaps, and other toiletries as well as in non-cosmetic products such as household cleaners and detergents. Its use worldwide is in the region of less than 0.01 metric tons per annum 17. 2-Hexadecene-1-ol and 3, 7, 11, 15-tetramethyl are a member of the fragrance structural group alcohols branched chain unsaturated. Their common characteristic structural elements are one hydroxyl group per molecule, a C4 to C16 carbon chain with one or several methyls or ethyl side chains and up to four non-conjugated double bonds. This individual fragrance material review is not intended as a stand-alone document. Please refer to A safety assessment of alcohols with the unsaturated, branched chain when used as fragrance Ingredients for an overall assessment of this material 18.
TABLE 1: CHEMICAL COMPOSITION OF THE ESSENTIAL OIL OF EQUISETUM ARVENSE
Phthalic acid and phthalate derivatives are major industrial materials used to manufacture plastic products like toys and bottles, also being widely used as plasticizers, adhesives, films, polymers, etc. However, some studies revealed that these compounds could affect the male reproductive systems producing testicular atrophy 19 and alter the normal development of fetuses in pregnant rats 20. In view of these adverse effects on living beings, the US Environmental Protection Agency (EPA) classified the phthalic acid and some industrial phthalates as priority pollutants 21 and so, for example, the maximum admissible content in water for a common phthalic acid derivative such as the di(2-ethylhexyl)phthalate was established in 6 g dm-3.
Gibberellic acid is a hormone present in higher plants that regulate different growth processes, with agricultural applications 22. Gibberellic acid plays an important role in many essential plant growth and development processes, including seed germination, stem elongation, leaf expansion, and reproductive development. Gibberellic acid is widely regarded as a growth promoting compound that positively regulates processes such as seed germination, stem elongation, leaf expansion, flower and fruit development, and floral transition.
The chemical composition of the total methyl esters of fatty acids from the extracts of Equisetum arvense showed to have a very similar profile. Fatty acid methyl esters are an alternative diesel fuel (namely, biodiesel) derived from vegetable oils or animal fats 23. The main components of vegetable oils and animal fats are triglycerides or also known as esters of fatty acids attached to a glycerol. The most widely used industrial method for the commercial production of fatty acid methyl esters from vegetable oils/fats is a base-catalyzed transesterification process using sodium hydroxide or potassium hydroxide as the homogeneous catalyst and methanol as the lower alcohol 24.
CONCLUSION: The source of many plants can often be identified from the peak pattern of the chromatograms obtained directly from GC-MS analysis. Similarly, unique qualitative and quantitative patterns from a GC analysis will often help identify the source of many alcoholic beverages. The technique of fingerprint could identify the false herbal products. The construction of chromatographic fingerprints aims at evaluating the quality of herbal medicines. The fundamental reason of quality control of herbal medicines is based on the concept of phytoequivalence of herbs, and then to use this conception to identify the real herbal medicine and the false one, and further to do quality control. The importance of the study is due to the biological activity of some of these compounds. The present study, which reveals the presence of components in Equisetum arvense suggest that the contribution of these compounds on the pharmacological activity should be evaluated.
ACKNOWLEDGEMENT: We are thankful to Vedat Akba (Criminal Police Laboratory, 25060, Erzurum, Turkey) for their help in GC-MS analyses. Also, the authors would like to thank to Melisa Demiray who helped in the collection of plants.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Kumar V and Parmar NS: Herbs: A potential source for the Development of new phytomedicines. The Pharma Review 2003; 1: 59-63.
- Mukherjee PK: Quality control of herbal drugs. An approach to the evaluation of botanicals. Business Horizons Pharmaceutical Publishers 2002.
- Clute WN: The fern allies of North America north of Mexico. Joliet, IL, Willard N. Clute & Co 1928; 278.
- Great Plains Flora Association. Flora of the Great Plains. Lawrence, KS, University Press of Kansas 1986.
- Navdeep SS, Sarabjit K and Divneet C: Pharmacognostic evaluation of equisetum arvense linn. International Journal of PharmTech Research 2010; 2: 1460-1464.
- Neda MD, Natasa S, Jelena C, Emilija J, Dejan O and Biljana B: Phenolic compounds in field horsetail (Equisetum arvense) as natural antioxidant. Molecules 2008; 13: 1455-1464.
- Wright CI, Van-Buren L, Kroner CI and Koning MM: Herbal medicines as diuretics: a review of the scientific evidence. Journal of Ethnopharmacology 2007; 114: 1-31.
- Corletto F: Female climacteric osteoporosis therapy with titrated horsetail (equisetum arvense) extract plus calcium (osteosil calcium): randomized double blind study. Miner Ortoped Traumatol 1999; 50: 201-206.
- Fabrício Hoffmann MDM, Jair G, Michael R, Vanusa M, Luzia K and Geanne MAC: Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense in mice. Pharmacological Research 2004; 49: 239-243.
- Dragana CS, Jasna MCB, Gordana MB, Sonja MD, Gordana SC, Vesna TT and Bratislav TS: Antioxidative and antiproliferative activities of different horsetail (Equisetum arvense) extracts. Journal of Medicinal Food 2010; 13: 452-459.
- Niko R, Gordana S and Radosav P: Composition and antimicrobial activity of Equisetum arvense essential oil. Phytotherapy Research 2006; 20: 85-88.
- Fathi-azad F and Lotfipour F: Study on the in-vitro antimicrobial activity of Achillea millefolium and Equisetum arvense. Pharmacology Sciences 2004; 1: 37-46.
- Daiana G, Esther G, Antonio JR, Vicente S and Sonia M: Mould growth and mycotoxin production as affected by Equisetum arvense and Stevia rebaudiana Food Control 2011; 8: 1378-1384.
- Oh H, Kim DH, Cho JH and Kim YC: Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. Journal of Ethnopharmacology 2004; 95: 421-424.
- Safiyeh S, Fathallah FB, Vahid N, Hossine N and Habib SS: Antidiabetic effect of Equisetum arvense (Equisetaceae) in streptozotocin-induced diabetes in male rats. Pakistan Journal of Biological Sciences 2007; 1: 1661-1666.
- Amit S, Saraswati B, Kamalesh U and Kumud U: Formulation and evaluation of a novel herbal gel of equisetum arvense extract. Journal of Pharmacognosy and Phytochemistry 2013; 1: 80-86.
- McGinty D, Letizia CS and Api AM: Fragrance material review on 2-hexadecen-1-ol, 3, 7, 11, 15-tetramethyl, Food and Chemical Toxicology 2010; 48: S101-S102.
- Belsito D, Bickers D, Bruze M, Calow P, Greim H, Hanifin JH, Rogers AE, Saurat JH, Sipes IG and Tagami H: A safety assessment of alcohols with the unsaturated, branched chain when used as fragrance ingredients. Food and Chemical Toxicology 2010; 48: S151-S192.
- Oishi S and Hiraga K: Testicular atrophy induced by phthalic acid esters: Effect on testosterone and zinc concentrations, Toxicology and Applied Pharmacology 1980; 53: 35-41.
- Makoto E, Emiko M, Akira H and Kunio K: Developmental toxicity evaluation of phthalic acid, one of the metabolites of phthalic acid esters, in rats. Toxicology Letters 1997; 93: 109-115.
- The United States Environmental Protection Agency (7407), Pollution Prevention and Toxics, EPA 749-F-95-016a, 1994.
- Tudzynski B: Biosynthesis of gibberellins in gibberella fujikuroi: biomolecular aspects. Applied Microbiology and Biotechnology 1999; 52: 298-310.
- Vasudevan PT and Briggs M: Biodiesel production current state of the art and challenges. The Journal of Industrial Microbiology and Biotechnology 2008; 35: 421-430.
- Semwal S, Arora AK, Badoni RP and Tuli DK: Biodiesel production using heterogeneous catalysts. Bioresource Technology 2011; 102: 2151-2161.
How to cite this article:
Yilmaz S, Ekinci S and Yilmaz B: Determination of bioactive compounds of Equisetum arvense by gas chromatography-mass spectrometry method. Int J Pharmacognosy 2014; 1(3): 184-88. doi: 10.13040/IJPSR.0975-8232.1(3).184-88.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
184-188
518
2782
English
IJP
S. Yilmaz, S. Ekinci and B. Yilmaz *
Department of Analytical Chemistry, Faculty of Pharmacy, Ataturk University, Erzurum, Turkey.
yilmazb@atauni.edu.tr
07 December 2013
19 February 2014
28 February 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(3).184-88
01 March 2014