ISOLATION AND CHARACTERIZATION OF SALACIA CHINENSIS AND ITS EVALUATION OF ANTIOXIDANT ACTIVITY
HTML Full TextISOLATION AND CHARACTERIZATION OF SALACIA CHINENSIS AND ITS EVALUATION OF ANTIOXIDANT ACTIVITY
A. R. Tamboli * 1 and A. G. Namdeo 2
Department of Pharmacognosy 1, Annasaheb Dange College of Pharmacy, Ashta - 416301, Maharashtra, India.
Department of Pharmacognosy, Poona College of Pharmacy, Bharati Vidyapeeth (Deemed to be University), Erandwane, Pune - 411038, Maharashtra, India.
ABSTRACT: Salacia chinensisis, commonly known as Saptarangi in the Hindi family of Hippocrateaceae, it is a woody climbing shrub found in Africa, Vietnam, and Thailand. A large number of biologically active compounds like salacinol, kotalanol, neokotalanol, neosalacinol, salasol, and mangiferin are isolated from S. chinensis. Traditionally, the plant is used in the treatment of diabetes, but there are few studies that demonstrate its use as anti-inflammatory, nephroprotective, anticancer, and treatment of cardiac disorders. The present study involves extraction, isolation, structural elucidation, and prediction of antioxidant activity from the roots of S. chinensis. The roots were extracted with water and methanol by using a hot extraction method. The methanol extract was fractionated with ethyl acetate. The antioxidant activity of different extracts was determined by 1, 1-diphenyl-2- picryl- hydrazyl (DPPH) method. The highest antioxidant activity was found in ethyl acetate extract, followed by methanol extract and water extract. Ethyl acetate extract showed maximum antioxidant activity, so the extract was used for the isolation of antioxidant compounds by column chromatography. The compound was isolated from the Salacia chinensis with higher yield and new technique. The compound isolated was characterized as 25, 26-oxido friedelane 1, 3-dione, and was elucidated using 1H NMR, 13C NMR, and MS. The study shows that the obtained pure compound could be a good source of natural antioxidants.
| Keywords: |
Salacia chinensis, Isolation, 25, 26-oxido friedelane 1, 3-dione, Chromatography spectroscopy, Antioxidant activity
INTRODUCTION: Large numbers of bioactive compounds are produced by plants that are used as an herbal medicine for the treatment of diseases since ancient times. Various phytochemicals, namely polyphenols, flavonoids, terpenes, phenolic acids, tannins, and coumarins, are present in plant and high concentrations of these phytochemicals may protect against free radical damage 1, 2.
The plants consist of beneficial phytochemicals, which is a need of the human body, and these phytochemicals act as natural antioxidants and source of supplementation for human diseases 3, 4. Antioxidants are considered a crucial chemical component, which may be responsible for preventing and delaying various types of cell damage.
The presence of antioxidants enhances the action of the immune system by producing a free radicle that is considered as one of the essential roles 5. It was observed that flavonoids and phenols are considered as strong antioxidants, and these are found to be distributed amongst various parts of plants.
The main aim of this study was to screen plant material extracts of finish origin with respect to antioxidant activity in order to find new potential sources of natural antioxidants. Different methods are used to evaluate the in-vitro antioxidant capacity of isolated compounds as well as those compounds containing different mixtures, which includes different mechanisms for the determination of antioxidant capacity of plant extract 2, 6. Salacia chinensis is an essential genus consisting of nutritional, medicinal, and pharmaceutical values belonging to the family of Hippocrateaceae. It is widely distributed across India, Sri Lanka, China, Myanmar, Thailand, Vietnam, and other Asian countries. Twenty-one species are found in India alone 7.
In India, S Chinensis is abundantly found in Karnataka, Goa, and Maharashtra. It is also called as, Saptarangi, Dimal, Modhupal, Ingli, Cherukuranti, Nisul-bondi and Eka Nayaka in Kannada. It is spread throughout the coast of the Andaman and Nicobar Islands and is a small erect or straggling tree or large, woody, climbing shrub 8. The S. chinensis tree can grow up to 3-10 m in height and 16 cm in diameter in the tropical forests 9. The species of S. Chinensis have medicinal value with high pharmacological significance. In the traditional system, it is used as acrid, bitter, thermogenic, urinary, and as a liver tonic. Extensive use has been reported in Ayurvedic system of medicine, traditional Indian medicine, and Unani for treating diabetes, gonorrhea, rheumatism, itching, asthma, ear diseases, leukemia, and inflammations 10.
Pharmacologically they are used in diseased conditions like respiratory disorder, chronic fever, cold, cough, malaria, dysentery, diarrhea, arthritis, skin diseases, convulsions, diabetes, trauma, and in treatment of internal organs, hepatic, vessel and immunologic disorders, Liver tonic and back pain 11. Salacia chinensis possess phytoconstituents like alkaloids, glycosides, polyphenols, coumarins, proteins, carbohydrates, gums and mucilage, fixed oil and volatile oil. The principle constituent like salacinol, kotalanol, salaprinol and ponkoranol, mangiferin is present in roots and stems and have been shown to be vital intestinal α-glucosidase inhibitors, and it lowers the absorption of carbo-hydrates in gastrointestinal tract and ultimately aims in delaying a rise in blood sugar by playing an essential role as anti-diabetic drug 12. Phyto-constituents are divided according to different parts of plant, phenolic glycosides, foliachineno-sides A1, A2, A3, B1, B2, C, and D are found to be present in leaf 13, Friedel-1-en-3-one; friedelane-1, 3-dione; 1, 3-dioxofriedelan-24-al and 7α- hydro-xyfriedelane-1,3-dione and 25, 26-oxido-friedel-1, 3-dione are present in root bark 14, 15. Salasones D; salasones E; salaquinone B; salasol B are found in the stem.
Morikawa et al., suggested the presence of wide variety of Triterpenoids like Friedelane-type triterpenes, salasones A, B, and C, norfriedelane-type triterpene, salaquinone A, acylated eudes-mane-type sesquiterpine, salasol A, 3β 22β- dihydroxyolean -12-en-29-oic acid; tingenone; tingenine B; regeol A; triptocalline A are found to be present in stem 16. However, not much literature is available on the antioxidant activity of isolated compounds obtained from Salacia species, which shows that no work has been carried out. Therefore, it is necessary to evaluate the antioxidant and biological activity of various isolated compounds. The present investigation involves isolating and characterizing antioxidant compounds from crude extract to determine their antioxidant activity.
FIG. 1: ROOT OF SALACIA CHINENSIS
MATERIALS AND METHODS:
Chemicals and Reagents: The solvents like hexane, ethyl acetate, methanol, chloroform, and acetone were used in this experiment obtained from Rankem Company, India. Silica gel, TLC silica gel aluminum sheets obtained by Merck Life Sciences, BHT (Butylated hydroxytoluene) and 1, 1-diphenyl-2-picrylhydrazyl (DPPH) obtained from Himedia Laboratories Pvt. Ltd., Mumbai. All glassware used in this experiment were from borosil, India. In addition, other chemicals used were of analytical grade.
Instruments: The absorbance of extracts and isolated pure compounds at different concentrations were measured by UV–visible spectroscopy (V630 JASCO, Japan) for the determination of antioxidant activity. The measurement of 1H and 13C NMR spectra were carried on Brucker (500 MHz) spectrometer (Pune University, Pune) using TMS as an internal standard. The measurement of FT-IR (4600 JASCO, Japan) was performed by using standard potassium bromide. Mass spectra were recorded on an Agilent 6460Triple Quadrupole LC/MS system and rota evaporator (BUCHI, model I200, Switzerland) used for the concentration of different solvents.
Sample Collections: The roots of Salacia Chinensis were sourced from Mayurbhanj, Orissa. A voucher specimen was collected from the source populations and was identified and authenticated by taxonomist Dr. P. Santhan. The herbarium sheets are maintained at R & D center, Sava Healthcare Ltd, Pune, India.
Extraction: The dried root samples of Salacia chinensis were grounded into coarse powder and the powdered samples 3 kg were further extracted with methanol 3 volume by using a hot extraction 60 ºC method for four hours 3 cycles.
The extraction was followed by the evaporation of the solvent by using a rotary evaporator under reduced pressure. Further, the solvent-free extract 500 g was fractionated using ethyl acetate 1500 ml. Solvent-free ethyl acetate extract 303 gm was again obtained by evaporating the solvent using a rotary evaporator.
Further ethyl acetate fraction (300 gm) was washed with (25% ethyl acetate: 75% Hexane) 900 ml. The following resulted in two parts; one was soluble 199 g, and the other obtained was insoluble 95 g. Then, the obtained soluble part was washed with hexane for reducing its non-polar impurity, after washing it with hexane the insoluble fraction 150 g was used for column chromatography.
Also, the fresh sample was extracted with water 3 volume by using a hot extraction of 60 ̊C methods for four hours 3 cycles. The resulting extracts of water, methanol, and ethyl acetate from root powder of S. chinensis were then subjected to their antioxidant activity.
Antioxidant Activity: The scavenging activity on α, α-diphenyl-β-picrylhydrazyl (DPPH) free radical was evaluated by the method of Shimada et al., 17 with slight modification. 1 ml of extract (at concentrations of 100, 200, 300, 400 or 500 μg/mL) was taken and to that, DPPH solution (1 mL, 0.1 mM in 95% ethanol) was added. The mixture was shaken and subjected at room temperature for 30 min. The absorbance of the resulting solution was measured at 517 nm. 1 ml of distilled water was used instead of sample for blank and sample control was prepared for each fraction by mixing 1 ml of sample with 1ml of 95% ethanol. BHT was used as a reference at a concentration of 200 μg/mL. Further, the radicle scavenging activity was calculated 18:
DPPH radical scavenging capacity (%) =1 − (Abs. of sample − Abs. of sample control) × 100/ Abs. blank
Isolation and Characterization Antioxidant Compounds: A different organic solvent like ethyl acetate and hexane was used as a mobile phase for the separation of ethyl acetate extract by using column chromatography. As per the same Rf value, the fractions obtained from column chromatography were mixed together, and the mother solvent was evaporated using a rota evaporator. According to TLC, the similar TLC pattern was integrated to give Fraction 1, Fraction 2, Fraction 3, Fraction 4, Fraction 5, and Fraction 6. Different polar solvents were used for the separation of ethyl acetate extract by using chromatographic techniques. The fractions were subjected to rotary evaporation at ambient temperature. From all the mentioned fractions, only Fraction 5 depicts a single band in TLC. The structural elucidation of the compound is carried out by using MS, IR, and 2D NMR 19, 20.
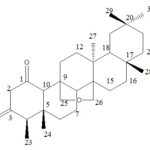
Fraction 5: The Fraction 5 obtained from ethyl acetate extract by column chromatography in which the stationary phase was silica gel (60-120 mesh) hexane and ethyl acetate of different polarities were used as mobile phase. The obtained fraction was found to be colorless crystals (4 g) Rf value 0.64 (ethyl acetate-hexane; 7:3); M.P 287 °C. The IR spectrum showed the presence of two carbonyl groups at 1732 and 1705 cm-1 characteristic diketone system. 1H NMR 500 MHz spectrum indicated three AB coupling doublets. The pair at 3.49 (1H, J 16), 3.27 (1H, J 15.9) is assigned to the methylene (C-2) of the β-diketone system. C-4 methine gives rise to a quartet at 2.57(1H, J 6.6) and a doublet due to the methyl at C-23 at 1.07 (3H, J 6.9). Five singlets due to five methyl’s are between 0.66-1.05. The C-10 methine gives rise to a singlet at 2.45. The doublet pairs at 4.57(1H, J 11.45), 4.02 (1H, J 11.25), 4.31 (1H, J 12), and 3.35 (1H, J12) are assigned to protons of the group -CH2-O-CH2- i.e., H-26a, H-26b, H25a, and H-25b respectively. The 13C NMR spectrum of compound indicated the presence of 30 carbons and suggested a triterpenoid structure. (M+, 454); On the basis of 1H and 13C and MS spectral data, it was characterized 25, 26-oxido friedelane 1, 3-dione Fig. 3 19, 20.
TABLE 1: 13C NMR DATA OF 25, 26-OXIDO FRIEDELANE 1, 3-DIONE
| Carbon no | Chemical shift | Carbon No | Chemical shift | Carbon no | Chemical shift |
| 1 | 202.8 | 11 | 35.8 | 21 | 32.5 |
| 2 | 60.5 | 12 | 26.5 | 22 | 38.9 |
| 3 | 230.7 | 13 | 38.1 | 23 | 7.5 |
| 4 | 59.7 | 14 | 40.0 | 24 | 15.7 |
| 5 | 37.5 | 15 | 34.7 | 25 | 67.1 |
| 6 | 38.5 | 16 | 36.8 | 26 | 69.9 |
| 7 | 17.0 | 17 | 30.8 | 27 | 19.3 |
| 8 | 45.2 | 18 | 44.0 | 28 | 30.0 |
| 9 | 37.1 | 19 | 35.4 | 29 | 31.4 |
| 10 | 69.0 | 20 | 28.3 | 30 | 35.1 |
RESULT: Different solvents like water, methanol, and ethyl acetate were used for the preparation of crude extracts of Salacia root, and the yield obtained is given in Table 2.
Antioxidant Activity: The antioxidant activity of different fractions of Salacia chinensis was evaluated by the DPPH method with modification. The highest antioxidant activity was found in ethyl acetate fraction with a percentage of inhibition value of 96.4, methanol fraction with percentage inhibition of 95.7, followed by water with percentage inhibition of 93.8 Table 3. The percentage inhibition of standard BHT 200 ug/ml was found to be 90%, which shows that the fractions of the selected plant species also show significant inhibition compared to standard BHT.
FIG. 2: TLC OF ISOLATED COMPOUND
Isolation and Characterization of Antioxidant Compounds: The highest antioxidant activity was found in ethyl acetate extract; the separation of ethyl acetate extract was done by column chromatography by the different polarity of solvents. The Fraction 5 obtained from the column chromatography gives a single band in TLC Fig. 2, which indicates the fraction is pure. The structure of the pure compound was elucidated by using IR, MS, and NMR.
Antioxidant Activity of Pure Compounds: The antioxidant activity of the isolated pure compound at different concentrations was determined by the same modified DPPH method. The pure compounds showed a significant percentage of inhibition at all applied concentrations against the DPPH method presented in Table 4.
DISCUSSION: There is no proven data available on the antioxidant activity of different solvent extracts like water, methanol, and ethyl acetate of the selected plant. The antioxidant activity of the crude extracts was determined by the DPPH method, and the results are presented in Table 3. The highest antioxidant activity was found in ethyl acetate extract, followed by methanol and water. Due to the presence of remarkable antioxidant activity, ethyl acetate crude extract was selected for isolation and separation of antioxidant compounds.
TABLE 2: YIELD OF DIFFERENT POLARITY OF CRUDE EXTRACTS OF SALACIA CHINENSIS
| Crude extract | Yield (g) |
| Methanol | 501 |
| Water | 523 |
| Ethyl acetate | 303 |
TABLE 3: ANTIOXIDANT POTENTIAL OF METHANOL, WATER AND ETHYL ACETATE CRUDE EXTRACTS OF S. CHINENSIS
| Fraction | Concentration (µg/ml) | Absorbance of sample control | Absorbance of Sample | % Inhibition |
|
Methanol fraction |
100 | 0.008 | 0.470 | 53.8 ± 0.02 |
| 200 | 0.031 | 0.382 | 64.9 ± 0.02 | |
| 300 | 0.052 | 0.254 | 79.8 ± 0.01 | |
| 400 | 0.063 | 0.123 | 94 ± 0.02 | |
| 500 | 0.069 | 0.112 | 95.7 ± 0.03 | |
| Water | 100 | 0.008 | 0.501 | 50.7 ± 0.18 |
| 200 | 0.031 | 0.410 | 62.1 ± 0.16 | |
| 300 | 0.052 | 0.303 | 74.9 ± 0.02 | |
| 400 | 0.063 | 0.189 | 87.4 ± 0.002 | |
| 500 | 0.069 | 0.125 | 93.8 ± 0.01 | |
| Ethyl acetate fraction | 100 | 0.008 | 0.455 | 55.3 ± 0.04 |
| 200 | 0.031 | 0.363 | 66.8 ± 0.03 | |
| 300 | 0.052 | 0.205 | 84.7 ± 0.01 | |
| 400 | 0.063 | 0.110 | 95.3 ± 0.02 | |
| 500 | 0.069 | 0.105 | 96.4 ± 0.02 |
TABLE 4: ANTIOXIDANT POTENTIAL OF PURE COMPOUND
| Fraction
|
Concentration (µg/ml) | Absorbance of Standard | Absorbance of Sample | %
Inhibition |
| 25,26-oxido friedelane 1,3-dione | 100 | 0.008 | 0.365 | 65.2 ± 0.03 |
| 200 | 0.031 | 0.199 | 83.2 ± 0.01 | |
| 300 | 0.052 | 0.116 | 95.3 ± 0.02 | |
| 400 | 0.063 | 0.099 | 96.4 ± 0.02 | |
| 500 | 0.069 | 0.093 | 97.6 ± 0.02 |
Various separation techniques such as thin-layer chromatography (TLC), column chromatography (CC) were used for the separation of compounds from the plant extract. In column chromatography, the mobile phase of different polarity was used for separation of compounds, and silica gel (120-60 mesh) was used as a stationary phase. Firstly, in the performed experiment mobile phase, hexane-ethyl acetate (95:5) was used, and a gradual increase in polarity of the mobile phase was done by the addition of ethyl acetate. In the isolation of pure compounds from crude extracts, polarity plays an important role. The TLC behavior shows that fraction 5 contains a single band as compared to the other fractions of ethyl acetate.
The pure compound obtained had colorless crystals. It had the molecular ion peak at [M]+ m/z 454, which corresponds to molecular formula C30H46O3. In the 1H NMR spectrum, the isolated compound shows three doublets, first doublet at d 3.49 and d 3.27, which indicates 2 protons at the position of C-2. Another doublet pairs at d 4.56, d 4.02, d 4.30, and d 3.35 are assigned to protons of the group -CH2-O-CH2- i.e., H-26a, H-26b, H25a, and H-25b respectively. C-4 methine gives rise to a quartet at d 2.57. Five singlets due to five methyls are seen between d 0.66- d 1.05.
The C-10 methine gives rise to a singlet at d 2.45 and a doublet due to the methyl at C-23 at d 1.07. Based on the above spectral data, the structure of the compound was established as 25, 26-oxido friedelane 1, 3-dione. The presence of two carbonyl groups at 1732 and 1705 cm-1 characteristic diketone system was shown by the IR spectrum.
The IR spectrum showed the presence of 2 carbonyl groups at 1732 and 1705 cm-1 characteristic diketone system. The compound was isolated with a new technique for the first time from the roots of Salacia chinensis with a higher yield. The TLC was carried out, and according to the TLC behavior, the compound was found to be almost pure.
FIG. 3: 25, 26-OXIDO FRIEDELANE 1, 3-DIONE
FIG. 4: 1H NMR SPECTRUM OF PURE COMPOUND
FIG. 5: 13C NMR SPECTRUM OF PURE COMPOUND
Antioxidant Activity of Pure Compound: The evaluation of antioxidant activity of the isolated pure compound 25, 26-oxido friedelane 1, 3-dione showed higher antioxidant activity against DPPH as compared to standard Butylated hydroxytoluene. The pure isolated compound shows the highest antioxidant activity as compared to water, methanol, and ethyl acetate extracts of Salacia chinensis.
CONCLUSION: Waste substances called free radicals are produced by cells as the body processes food and reacts to the environment. If the body is unable to process and remove free radicals, it will result in oxidative stress. This oxidative stress is directly connected to heart disease, cancer, arthritis, stroke, respiratory diseases, immune deficiency, emphysema, Parkinson's disease, and other inflammatory or ischemic conditions.
Role of antioxidants is to neutralize free radicals in our body, which boosts overall health. Therefore, the risks of certain diseases are lowered due to antioxidants. Salacia chinensis is a medicinal plant, which contains high levels of antioxidant activity 21, 22. The ethyl acetate extract has the highest antioxidant activity so it was selected for isolation and separation of antioxidant compounds. The antioxidant activity of extracts was found in the order of ethyl acetate> methanol> water. The isolated compound 25, 26-oxido friedelane 1, 3-dione showed maximum antioxidant activity against DPPH as compared to standard BHT.
ACKNOWLEDGEMENT: I would like to record my gratitude to my esteemed respected guide Dr. A.G. Namdeo, Department of Pharmacognosy, Poona College of Pharmacy, Pune.
CONFLICTS OF INTEREST: The authors declare that there is no conflict of interests regarding the publication of this paper.
REFERENCES:
- Suffredini IB, Sader HS, Gonçalves AG, Reis AO, Gales AC, Varella AD and Younes RN: Screening of antibacterial extracts from plants native to the Brazilian Amazon Rain Forest and Atlantic Forest. Brazilian Journal of Medical and Biological Research 2004; 37(3): 379-84
- Hsu FL, Huang WJ, Wu TH, Lee MH, Chen LC, Lu HJ, Hou WC and Lin MH: Evaluation of antioxidant and free radical scavenging capacities of polyphenolics from pods of Caesalpinia pulcherrima. International Journal of Molecular Sciences 2012; 13(5): 6073-88.
- Boots AW, Haenen GR and Bast A: Health effects of quercetin: from antioxidant to nutraceutical. European Journal of Pharmacology 2008; 585(2-3): 325-37.
- López-Alarcón C and Denicola A: Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Analytica Chimica Acta 2013; 763: 1-0.
- Veerapur VP, Prabhakar KR, Kandadi MR, Srinivasan KK and Unnikrishnan MK: Antidiabetic effect of Dodonaea viscosa aerial parts in high fat diet and low dose streptozotocin-induced type 2 diabetic rats: a mechanistic approach. Pharmaceutical Biology 2010; 48(10): 1137-48.
- Shah NA, Khan MR, Naz K and Khan MA: Antioxidant potential, DNA protection and HPLC-DAD analysis of neglected medicinal Jurine adolomiaea Biomed Research International 2014; 726241: 10.
- Singh NP, Vohra JN, Hajra PK and Singh DK: Flora of India, Botanical Survey of India; 2000; 5: 150-162.
- Ghanam K, Srivastava V, Jayant Deshpande and Vijay Juturu, Salacia chinensis Extract (SCE) Modulates Carbohydrates and Lipid Metabolism: in-vitro and in-vivo Models, Endocrinology and Metabolism International Journal 2016; 3(6) : 2
- Chavan JJ, Ghadage DM, Bhoite AS and Umdale SD: Micropropagation, molecular profiling and RP-HPLC determination of mangiferin across various regeneration stages of Saptarangi (Salacia chinensis). Industrial Crops and Products 2015; 76: 1123-32.
- Matsuda H, Yoshikawa M, Morikawa T, Tanabe G and Muraoka O: Antidiabetogenic constituents from Salacia species (Chemical & Pharmacological study). Chinese Medicine Pharmacology 2005; 22(1): 145-53.
- Nadkarni AK and Nadkarni KM: Indian Materia Medica. Popular Prakashan Bombay 1976; 263-69.
- Yoshikawa M, Pongpiriyadacha Y, Kishi A, Kageura T, Wang T and Morikawa T: Biological activities of Salacia chinensis originating in Thailand: the quality evaluation guided by alpha-glucosidase inhibitory activity. Yakugaku Zasshi 2003; 123(10): 871-80.
- Nakamura S, Zhang Y, Pongpiriyadacha Y, Wang T, Matsuda H and Yoshikawa M: (a). Megastigmane Glycosides from the leaves of Salacia chinensis. Heterocycles 2008; 75: 131-43.
- Tewari NC, Ayengar KN and Rangaswami S: Structure of some crystalline components of Salacia prenoides. Current Science 1971; 40(22): 601-2.
- Rogers D, Williams DJ, Joshi BS, Kamat VN and Viswanathan N: Structure of a new triterpene ether from Salacia prinoides dc: x-ray investigation of the dibromo derivative. Tetrahedron Letters 1974; 15(1): 63-6.
- Morikawa T, Kishi A, Pongpiriyadacha Y, Matsuda H and Yoshikawa M: Structures of New Friedelane-Type Triterpenes and Eudesmane-Type Sesquiterpene and Aldose Reductase Inhibitors from Salacia chinensis. Journal of Natural Products 2003; 66(9): 1191-6.
- Shimada K, Fujikawa K, Yahara K and Nakamura T: Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry. 1992 Jun; 40(6):945-8.
- Surendraraj A, Farvin KHS and Anandan R: Antioxidant potential of water hyacinth (Eichornia crassipes): in-vitro antioxidant activity and phenolic composition. Journal of Aquatic Food Product Technology 2013; 22(10): 11-26.
- Gunatilaka AAL, Nanayakkara NPD and Wazeer MIM: 13c NMR spectra of some d:a-friedo-oleananes department of chemistry, University of Peradeniya, Sri Lanka; Organic Magnetic Resonance 1980; 15(5): 415-17
- Duarte LP, Figueiredo RC, de Sousa G, da Silva DBS, Rodrigues SBV and de Silva FCSDF: Chemical Constituents of Salacia elliptica (Celastraceae) Quim. Nova 2010; 33(4): 900-03.
- Ngo TV, Scarlett CJ, Bowyer MC and Vuong QV: Phytochemical and antioxidant properties from different parts of Salacia chinensis Journal of Biologically Active Products from Nature 2017; 7(5): 401-10.
- Chavanemailauthoru JJ, Gaikwadg JBB and Dixitv BA: Bapattotal phenolics, flavonoids and antioxidant activity of Saptarangi (Salacia chinensis L.) Fruit pulp. Journal of Plant Biochemistry and Biotechnol 2013; 22(4): 409-13.
How to cite this article:
Tamboli AR and Namdeo AG: Isolation and characterization of Salacia chinensis and its evaluation of antioxidant activity. Int J Pharmacognosy 2020; 7(5): 126-32. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(5).126-32.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
126-132
872
1186
English
IJP
A. R. Tamboli * and A. G. Namdeo
Department of Pharmacognosy, Annasaheb Dange College of Pharmacy, Ashta, Maharashtra, India.
amirrtamboli@gmail.com
03 April 2020
11 May 2020
20 May 2020
10.13040/IJPSR.0975-8232.IJP.7(5).126-32
31 May 2020