COMPOSITIONS, DISTRIBUTIONS AND STATUS OF ECONOMIC PLANTS AMONG INVASIVE FLORAS OF UTTARPARA, WEST BENGAL, INDIA
HTML Full TextCOMPOSITIONS, DISTRIBUTIONS AND STATUS OF ECONOMIC PLANTS AMONG INVASIVE FLORAS OF UTTARPARA, WEST BENGAL, INDIA
Saikat Biswas, Mayum Maity, Sounak Srimany, Subhashree Chatterjee, Tanaya Karmakar, Ruma Datta, Jhilam Patra, Mithun Koley and Dibyendu Talukdar *
Department of Botany, R.P.M. College (University of Calcutta), Uttarpara - 712258, West Bengal, India.
ABSTRACT: A survey was carried out from 2005 to 2010 to invent the invasive alien plant species and their economic uses in urban and adjoining rural areas of Uttarpara, Hooghly district, West Bengal. The study revealed the occurrence of 103 alien angiospermic plant species under 32 families, of which four families (Araceae, Poaceae, Cyperaceae, Pontederiaceae) are monocots. Dicot family Fabaceae dominated with 20 plant species, followed by Asteraceae with 17, Amaranthaceae with 8, Solanaceae with 7, Euphorbiaceae with 5, and then other families. Rise in number of alien species was evidenced in year-wise quadrat studies, screening 11 most invasive species namely Parthenium hysterophorus, Eupatorium odoratum, Ageratum conyzoides, A. haustonianum, Chromolaena odorata, Cassia sophera, Leucaena leucocephala, Alternanthera sessilis, Amaranthus spinosus, Lantana camara and tree, Trema orientralis distributed within Asteraceae (5 taxa), Fabaceae (2 taxa), Amaranthaceae (2 taxa), Verbenaceae (1 taxon) and Ulmaceae (1 taxon). Remarkably, the alien species have been used in diverse economic and commercial purposes by local village folks, showing the use of nearly 49% plants in local health-care systems as herbal products.
| Keywords: |
Invasive alien plants, Biodiversity, Uttarpara, Economic values
INTRODUCTION: Invasive alien species are colonizer species that have established populations outside their native distributional ranges and that have the potential to spread and affect native ecosystems or local human-mediated systems 1, 2. Biological invasions by alien species are widely recognized as the second worst threat to native biodiversity and impose high costs to agriculture, forestry, and aquatic ecosystems.
The global extent and rapid increase in invasive species are homogenizing the world’s flora and fauna 3 and is recognized as a primary cause of global biodiversity loss, diminishing regional distinctiveness of flora and fauna and catalyzing homogenization of biota 4, 5.
A large number of alien plants are daily used by the local population in fuel, sheltering, fishing, medicinal, and other purposes 6, 7. At least 10% of the world’s vascular plants (3,00,000) have the potential to invade other ecosystems and affect native biota in direct and indirect ways 8. About 18% of the Indian flora is composed of alien species, of which tropical. America has the largest share (55%), followed by Asia (30%), and Europe, the Mediterranean and others (15%).
About 40% of the Indian flora is alien, of which 25% are invasive alien species 8, 9. As India is considered as a biodiversity-rich country, authentic documentation of alien plant species is urgently needed at the regional level to get a comprehensive national database for better management and utilization of exotic floras 10. The state of West Bengal is located between 85˚ 50΄ and 89˚ 50΄ E and 21˚ 38΄ and 27˚ 10΄ N, and one of popular but the biodiversity-rich states in India. The lower Indo-Gangetic basin constitutes fertile hub for diverse types of flora and fauna, introduced by anthropogenic activities since time immemorial. The Hooghly district is an important part of this basin, dotted with numerous wetlands, forest covers, and agricultural lands. Hooghly is the major river with some small rivers constituting the riverine and floodplain systems in this district. Uttarpara (situated between 22˚40΄N and 22.67˚05΄N latitude and 88˚21' and 88˚35΄E longitude) is one of the oldest heritage sites in Hooghly district, delimited by Bally khal in South and river Hooghly in the eastern side representing lower part of Gangetic basin.
The climate of the district is tropical monsoon with three distinct seasons-summer (March-early June), rainy (June - September) and winter (October -February), and mean annual rainfall ca, 1300 mm. While maximum summer temperature may sore to 43 °C, winter is extremely chilled with temperature may plummet to 10-12 °C. The region is very rich in biodiversity, but extensive urbanization coupled with the introduction of exotic plant species cause a threat to native floras. As biological invasions are frequently influenced by ecosystem functioning 11, climate change 12-14, environmental pollution 15, and other physicochemical mechanisms, a proper first-hand inventory in disturbed areas is necessary to assess threats on indigenous resources.
Although, rich in floral diversity, no investigation was carried out to document the economic plant's utilizations among invasive alien plants in this area. In the last 5-6 years, activities of trade, various infrastructure development projects, vehicle, and rail transport increased to a considerable extent. As invasive species has huge ecological impacts and preference over native species in urban ecology due to their faster rate of growth, biomass production, allelopathic potential, high reproductive efficiency, seed dispersal types, rapid establishments and hardiness to abiotic stress, documentation of alien plants is necessary. The objectives of the present study are, therefore, to document the alien flora, their classification and use by local people in and around the Uttarpara region.
MATERIALS AND METHODS:
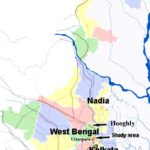
Study Site: The present investigation was carried out by extensive field survey during the last six years (2005-2010) in different intervals (March-June, September - January) in Uttarpara area covering both urban and villages adjacent it Fig. 1.
FIG. 1: A MAP OF STUDY AREAS (RED DOTS) IN UTTARPARA HOOGHLY DISTRICT, WEST BENGAL, INDIA
Collection of Data and Methods of Inventory: Plant samples were collected either in flowering or fruiting stage, and voucher specimens were deposited in departmental herbaria, R.P.M. College, Uttarpara, Hooghly and pressed specimens are being digitalized in Digital Phyto-Informatics Center of the Botany Department (http://www.rpmc digitalphytoinformatics.com/).
Invasive nature of some of the worst alien species, enlisted by previous works 16, 17, was studied using techniques of Baider and Florens 18, namely through a combination of random walks through the area along with a more quantitative sampling of the seedlings and larger woody plants (flowering or fruiting stage) in a series of square quadrats (1 × 1 m for seedlings and 10 × 10 m for tree). Frequency (F%) of particular plant species was calculated by dividing the number of quadrats in which a particular species occur with a total number of quadrats laid down. The specimens were identified through an extensive survey of available literature, monographic works, and confirmed by IPNI (International Plant Names Index) database (www. IPNI.org). Use of documented flora was tabulated through interviews of knowledgeable people like temple priests, the village head, old experienced folk, medicine men, farmers, teachers, etc. Structured questionnaires thoroughly cross-checked gathered information, and documented after that. Nativity of the species was tested from the available literature 19.
RESULTS:
Documentation and Classification of Alien Taxa: Present inventorization of the alien invasive flora in and around Uttarpara revealed the occurrence of 103 species belonging to 83 genera under 32 families Table 1. Among the plant growth form, herbs constituted 85%, and it was followed by shrub (9%), tree (4%) and climbers (2%). Several genera were found to possess three or more species Table 1.
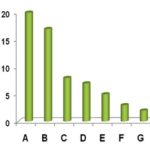
FIG. 2: DISTRIBUTION OF ALIEN PLANT SPECIES IN DIFFERENT ANGIOSPERM FAMILIES; A-Family Fabaceae, B-Asteraceae, C- Amaranthaceae, D-Solanaceae, E-Euphorbiaceae, F- Malvaceae / Scrophulariaceae / Lamiaceae / Convolvulaceae/ Asclepiadaceae/Poaceae (three species each), G-Cuscutaceae/ Verbenaceae/ Pontederiaceae/ Acanthaceae/ Polygonaceae (two species each), H-rest of the 16 families with one species each, as mentioned in Table 1.
Dicotyledonous species contributed a major proportion (94%) of alien flora grouped under 96 species and 28 families Table 1, followed by Monocotyledons (6%) distributed in seven genera under four families. Among the total 32 families, Fabaceae dominated with 20 species, followed by Asteraceae (17 species), Amaranthaceae (8), Solanaceae (7) and then others Table 1 and Fig. 2.
Habitat Distribution: About 38% of invasive species identified in the present study were most abundant in roadside (GT roads and rural roads) bushes, while inside area was suitable for 32% plant species. Cultivated fields and banks of water bodies were preferred by 20% and 10% species, respectively. Quadrat studies in the last six years revealed a high frequency of some of the daisies like Parthenium hysterophorus, Eupatorium odoratum, Ageratum conyzoides, Ageratum houstonianum, Chromolaena odorata along the roadside than the interior of the villages Fig. 3.
FIG. 3: VILLAGE-FOREST/ROADSIDES RATIO OF NUMBER OF PLANTS FOR ELEVEN ALIEN TAXA
The ratio of number of plants (cumulative of 400 quadrats / year in six years) between interior villages/forest area and roadside varied between 0.53-0.88 for these five daisy members, while it was close to 1.0 for Cassia sophera (0.98), >1.0 for Leucaena leucocephala, Alternanthera sessilis, Trema orientalis and Amaranthus spinosus, and was 2.15 for Lantana camara Fig. 3. By contrast, Wedelia Chinensis, Tridax procumbens and Eclipta prostrata exhibited higher frequency in the interior of the study sites (F=80-86%) than the roadside (F=65-72%).
Within the village area, members of Amaranthaceae such as Achyranthes aspera, Alternanthera philoxeroides, Alternanthera sessilis, and Amaranthus spinosus dominated intermingling with numbers of leguminous plants like Aeschynomene americana, different species of Cassia, Crotalaria, Leucaena leucocephala, Mimosa pudica, and species of other families in different magnitudes. Interestingly, the frequency of Parthenium reduced in plots where species of family Amaranthaceae dominated. The tree Trema orientalis flourished within the undisturbed area better than the roadside Fig. 3. Members of family Polygonaceae, Araceae, Cyperaceae, Ponte-deriaceae preferred wetland areas, while Solanaceae, Euphorbiaceae, Malvaceae, Cactaceae, Convolvulaceae and Asclepiadaceae were more frequent in a dry land.
Documentation of spread of alien flora over the last six years (2005-2010) revealed steep rise in number of certain plant species (quadrat wise) such as species of Parthenium, Ageratum, Chromolaena, Eupatorium, Cassia, Leucaena, Alternanthera, Lantana, Amaranthus and Trema Fig. 4, while low to moderate rise was documented for other species (data not presented).
FIG. 4: FREQUENCY % OF 11 INVASIVE PLANTS AS RECORDED FROM SIX CONSECUTIVE YEARS (2005-2010) WITH 400 SQUARE QUADRATS LAID DOWN/YEAR, A- Parthenium hysterophorus L., B- Eupatorium odoratum L., C- Ageratum conyzoides L., D- Ageratum houstonianum Mill., E- Chromolaena odorata (L.) King & Robinson, F- Leucaena leucocephala (Lam.) de Wit, G- Cassia sophera L., H- Alternanthera sessilis (L.) R.Br. ex DC, I- Lantana camara L, J- Trema orientralis (Linn.) Blume, and K- Amaranthus spinosus L.
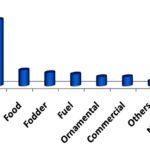
Economic / Commercial Utilization of Alien Plants by Local People: The 103 plant species documented as alien flora in the present inventory have been used by the local population as food, fodder, medicinal, ornamental, commercial (fishing, thatching, basket making, etc.), religious and other purposes Fig. 5, revealing resource utilization by people in diverse ways. Among the species, members of legume family Fabaceae have been used most extensively as food, fodder, fuel, manure, folk play and other purposes Table 1.
As local people revealed, wild beans (Phaseolus spp), mungs (Vigna spp), khesari (Lathyrus sativus L.) and jangli matar (Lathyrus aphaca L.) have considerable benefits in their daily life; seed flour as food supplement, making besans, pokaras, whole plant as fodder, soil fertilizer (mulching), and tender pod as vegetables. About 49% of total alien plants were used as medicinal purposes, while 11.6% of plants were utilized as food and 9.7% used as cattle feed.
Among the small-scale cottage industries, preparation of beads on the string using seeds of Coix (Poaceae) and commercial ‘shola’ using Aeschynomene americana (Fabaceae) were found highly beneficial for local economies. Different types of wood works, another financially viable activity within the study area, are mainly carried out with Prosopis julifera (Fabaceae) tree. Besides, different plant parts have been used as folk play, insecticide and aromatic purposes Table 1 and Fig. 5.
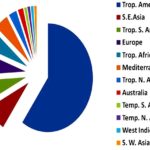
Nativity of Documented Alien Flora: The contribution of different geographical regions in terms of the nativity of documented flora was shown in Fig. 6. Tropical America accounted for nearly 60% plants, followed by a share of South-East Asia, Europe and tropical South America, tropical Africa, the Mediterranean, and other regions.
TABLE 1: INVASIVE PLANT SPECIES AND THEIR UTILIZATIONS IN UTTARPARA AREAS OF HOOGHLY DISTRICT OF WEST BENGAL, INDIA
| S. no. | Species | Family | Life form | Nativity | Use |
| 1 | Aerva javanica (Burm.f.)
Juss.ex Schult |
Amaranthaceae | Herb | Trop. America | M |
| 2 | Achyranthes aspera L. | Amaranthaceae | Herb | Trop. America | M |
| 3 | Aeschynomene Americana L. | Fabaceae | Herb | Trop. America | Co, ‘shola’ |
| 4 | Ageratum conyzoides L. | Asteraceae | Herb | Trop. America | NU |
| 5 | Ageratum houstonianum Mill. | Asteraceae | Herb | Trop. America | NU |
| 6 | Alternanthera philoxeroides
(Mart.) Griseb. |
Amaranthaceae | Herb | Trop. America | Veg |
| 7 | Alternanthera pungens Kunth | Amaranthaceae | Herb | Trop. America | M |
| 8 | Alternanthera sessilis (L.) R.Br. ex DC | Amaranthaceae | Herb | Trop. America | M, Veg |
| 9 | Amaranthus spinosus L. | Amaranthaceae | Herb | Trop. America | M |
| 10 | Argemone mexicana L. | Papaveraceae | Herb | Trop. America | NU |
| 11 | Bidens pilosa L. | Asteraceae | Herb | Trop. America | M |
| 12 | Blumea lacera (Burm.f.) DC. | Asteraceae | Herb | Trop. America | M, veg |
| 13 | Boerhaavia erecta L. | Nyctaginaceae | Herb | Trop. America | M, veg, Cf |
| 14 | Calotropis gigantea (L.) R.Br. | Asclepiadaceae | Shrub | Trop. Africa | M |
| 15 | Calotropis procera (L.) R.Br. | Asclepiadaceae | Shrub | Trop. Africa | M |
| 16 | Cassia alata L. | Fabaceae | Shrub | West Indies | M, Thatching |
| 17 | Cassia javanica L. | Fabaceae | Tree | S.E. Asia | Or, M |
| 18 | Cassia occidentalis L. | Fabaceae | Herb | Trop. S. America | M, Bf |
| 19 | Cassia sophera L. | Fabaceae | Herb | Trop. S. America | M, Bf |
| 20 | Catharanthus pusillus (Murray)Don | Apocynaceae | Herb | Trop. America | M |
| 21 | *Chromolaena odorata (L.) King & Robinson | Asteraceae | Herb | Trop. America | NU |
| 22 | Chrozophora rottleri (Geis.)
Spreng. |
Euphorbiaceae | Herb | Trop. Africa | M |
| 23 | Chenopodium album L. | Chenopodiaceae | Herb | Europe | Veg, Cf |
| 24 | Cleome gynandra L. | Cleomaceae | Herb | Trop. America | M |
| 25 | Cleome monophylla L. | Cleomaceae | Herb | Trop. America | M |
| 26 | Cleome rutidosperma DC. | Cleomaceae | Herb | Trop. America | M |
| 27 | Coix lacryma-jobi L. | Poaceae | Herb | S. E. Asia | Pearl, fishing |
| 28 | Crotalaria pallida Dryand | Fabaceae | Herb | Trop. America | Bf |
| 29 | Crotalaria retusa L. | Fabaceae | Herb | Trop. America | Bf |
| 30 | Croton bonplandianum Boil. | Euphorbiaceae | Herb | Temp.S. America | M |
| 31 | Cryptostegia grandiflora
R.Br. |
Asclepiadaceae | Woody Climber | Trop. Africa (Madagascar) | M |
| 32 | Cuscuta chinensis Lam. | Cuscutaceae | Herb | Mediterranean | NU |
| 33 | Cuscuta reflexa Roxb | Cuscutaceae | Herb | Mediterranean | NU |
| 34 | Cyperus rotundus L. | Cyperaceae | Herb | Africa, S. Europe | M |
| 35 | Cytisus scoparius (L.) Link | Fabaceae | Herb | Europe | M |
| 36 | Datura innoxia Mill. | Solanaceae | Shrub | Trop. America | M |
| 37 | Datura metel L. | Solanaceae | Shrub | Trop. America | M |
| 38 | Dentella repens (L.) Forst | Rubiaceae | Herb | E. Asia, Australia | Veg |
| 39 | Digera muricata (L.) Mart. | Amaranthaceae | Herb | S. W. Asia | Veg |
| 40 | Duranta repens L. | Verbenaceae | Shrub | Trop. America | Or |
| 41 | Echinochloa crusgalli (L.) P.Beauv. | Poaceae | Herb | Trop. S. America | M |
| 42 | Echinacea paradoxa Britton | Asteraceae | Herb | Europe | NU |
| 43 | Eclipta prostrata (L.) Mant. | Asteraceae | Herb | Trop. America | M |
| 44 | *Eichhornia crassipes kunth | Pontederiaceae | Aq. Herb | Trop. America | NU |
| 45 | *Eupatorium odoratum L. | Asteraceae | Herb | Europe | NU |
| 46 | Euphorbia hirta L. | Euphorbiaceae | Herb | Trop. America | Cf |
| 47 | Euphorbia heterophylla L. | Euphorbiaceae | Herb | Trop. America | Cf |
| 48 | Evolvulus nummularius L. | Convolvulaceae | Herb | Trop. America | Cf |
| 49 | Gnaphalium coarctatum Willd. | Asteraceae | Herb | Trop. America | NU |
| 50 | Gnaphalium pensylvanicum
Willd. |
Asteraceae | Herb | Trop. America | NU |
| 51 | Gomphrena serrata L. | Amaranthaceae | Herb | Trop. America | Or |
| 52 | Hyptis suaveolens (L.) Poit. | Lamiaceae | Herb | Trop. America | Aromatic |
| 53 | Impatiens balsamina L. | Balsaminaceae | Herb | Trop. America | Or |
| 54 | Indigofera astragalina DC. | Fabaceae | Herb | Trop. America | Cloth washing |
| 55 | Indigofera linifolia (L.f.) Retz. | Fabaceae | Herb | Trop. America | NU |
| 56 | Ipomoea quamoclit L. | Convolvulaceae | Herb | Trop. America | Bf |
| 57 | Ipomoea aquatica Forsk | Convolvulaceae | Aquatic | Trop. America | M, veg |
| 58 | *Lantana camara L. | Verbenaceae | Herb | Trop. America | Bf |
| 59 | Lathyrus aphaca L. | Fabaceae | Herb | Mediterranean | M, Cf, mulching |
| 60 | Lathyrus sativus L. | Fabaceae | Herb | Mediterranean | Pulse, Fd, besan, Cf, veg |
| 61 | Leonotis nepetiifolia (L.) R.Br. | Lamiaceae | Herb | Trop. Africa | M |
| 62 | *Leucaena leucocephala (Lam.) de Wit | Fabaceae | Tree | Trop. America | Bf, basket making, |
| 63 | Ludwigia perennis L. | Onagraceae | Herb | Trop. America | M |
| 64 | Malachra capitata (L.) L. | Malvaceae | Herb | Trop. America | M |
| 65 | Mecardonia procumbens (Mill.) Small | Scrophulariaceae | Herb | Trop. N. America | NU |
| 66 | Melilotus alba Desv. | Fabaceae | Herb | Europe | Insecticide |
| 67 | *Mikania micrantha Kunth | Asteraceae | Climber | Trop. America | NU |
| 68 | Mimosa pudica L. | Fabaceae | Herb | Trop. S. America | M |
| 69 | Monochoria vaginalis (Burm.f.)C. Presl. | Pontederiaceae | Aquatic herb | Trop. America | M |
| 70 | Nicotiana plumbaginifolia Viv. | Solanaceae | Herb | Trop. America | NU |
| 71 | Ocimum basilicum L | Lamiaceae | Herb | Trop. America | M |
| 72 | * Opuntia stricta (Haw.) Haw. | Cactaceae | Herb | Trop. America | NU |
| 73 | Oxalis corniculata (DC.) Raeusch. | Oxalidaceae | Herb | Europe | M |
| 74 | Parthenium hysterophorus L. | Asteraceae | Herb | Trop. N. America | NU |
| 75 | Pennisetum purpureum Schum. | Poaceae | Herb | Trop. N. America | Cf |
| 76 | Peperomia pellucida (L.) Kunth | Piperaceae | Herb | Trop. America | Folk play |
| 77 | Peristrophe paniculata (Forssk.) Brummitt | Acanthaceae | Herb | Trop. America | NU |
| 78 | Phaseolus aureus L. | Fabaceae | Herb | Trop. America | Fd, Cf, veg |
| 79 | Phyllanthus fraternus Webster | Euphorbiaceae | Herb | Trop. America | M |
| 80 | Physalis angulata L. | Solanaceae | Herb | Trop. America | Folk play |
| 81 | Pilea microphylla (L.) Liebm. | Urticaceae | Herb | Trop. America | NU |
| 82 | Pistia stratiotes L. | Araceae | Herb | Trop. America | M |
| 83 | Polygonum barbatum L. | Polygonaceae | Herb | S. E. Asia | M |
| 84 | Polygonum hydropiper L. | Polygonaceae | Herb | S. E. Asia | M |
| 85 | Portulaca oleracea L. | Portulacaceae | Herb | Trop. S. America | Or |
| 86 | Prosopis juliflora (Sw.) DC. | Fabaceae | Tree | Trop.S. America | Wood works |
| 87 | Ruellia tuberosa L. | Acanthaceae | Herb | Trop. America | Or |
| 88 | Scoparia dulcis L. | Scrophulariaceae | Herb | Trop. America | M |
| 89 | Sesbania grandiflora | Fabaceae | Shrub | Trop. America | Bf, veg, M, Or |
| 90 | Sida acuta Burm.f. | Malvaceae | Herb | Trop. America | M |
| 91 | Solanum torvum Sw. | Solanaceae | Shrub | Trop. America | M |
| 92 | Solanum xanthocarpum | Solanaceae | Shrub | Trop. America | NU |
| 93 | Solanum nigrum L. | Solanaceae | Herb | Trop. America | M |
| 94 | Sonchus oleraceus L. | Asteraceae | Herb | Mediterranean | NU |
| 95 | Spilanthes radicans Jacq. | Asteraceae | Herb | Trop. America | M |
| 96 | Tephrosia purpurea (L.) Pers., | Fabaceae | Herb | Trop. America | M |
| 97 | Torenia fournieri Linden ex E. Fournier | Scrophulariaceae | Herb | Australia | NU |
| 98 | Trema orientralis (Linn.)
Blume |
Ulmaceae | Tree | S. E. Asia | M, Bf, fishing, thatching |
| 99 | Tridax procumbens L. | Asteraceae | Herb | Trop. America | M |
| 100 | Urena lobata L. | Malvaceae | Herb | Trop. America | M |
| 101 | Wedelia chinensis | Asteraceae | Herb | S. E. Asia | M |
| 102 | Vernonia cinera L. | Asteraceae | Herb | Temperate America | NU |
| 103 | Vigna sublobata (L.)
Wilczek |
Fabaceae | Herb | S. E. Asia | Pulse, ‘bori,’ besan, Cf |
* enlisted in the database of world’s 100 worst invasives; M-medicinal, Co-compost, Or -ornamental, Bf-biomass fuel, Cf-cattle feed, Fd-Food, Veg-Vegetables, NU-not in use.
FIG. 5: UTILIZATION OF RESOURCES OF ALIEN PLANTS BY LOCAL PEOPLE; OTHERS INCLUDE FOLK PLAY, AROMATIC, INSECTICIDE
FIG. 6: NATIVITY OF INVESTIGATED 103 ALIEN TAXA IN 12 WORLD GEOGRAPHIC REGIONS WITH LION’S SHARE IS FROM TROPICAL (TROP) AMERICA, S-SOUTH, E-EAST, N-NORTH, W-WEST
DISCUSSION: In the present inventory, the share of invasive species with economic utilization in the floral compositions of study areas has been estimated to 57.11%, while rest of the amount was made up of native species, suggesting the dominance of alien flora in Uttarpara areas. Herbs constituted major portions (85%) of this alien species, and except only seven species, all others belong to 28 different dicot families. This suggests that increasing urbanization in and around Uttarpara has coincided with an increase in alien plant population.
The dominance of leguminous (Fabaceae) plants in the present study over other species agrees well with earlier reports on Sub-Himalayan North Bengal, India 7. Although, Fabaceae contained the highest number of alien species in the present study, the dominance of Asteraceae in invasive alien flora was reported in parts of India 19, China 18, 20, and other countries 21. The higher frequency of some asters along the roadside than the interior of the village / bushes indicated the uneven distribution of Asteraceae weeds in the present study area, resulting in the interior village/roadside ratio in the number of plants below 1.0 for the family. Unlike asters, members of Fabaceae and Amaranthaceae were more evenly distributed as revealed by quadrat plots. The higher number of Lantana camara and Trema orientralis in forest wasteland suggested an invasion of these two aggressive species in nutrient-deficient regions in the expense of native flora, leading to village/ roadside ratio for these two species over 1.0.
The relative degree of disturbances in habitats, apart from abiotic and biotic constraints, have a profound effect of changing the physical environment, creating opportunities for intro-duction and establishment of non-indigenous species to invade native systems in this forest area, as argued in other invasive biological systems 6, 18.
Reduction in frequency of one of the world’s worst noxious weeds, Parthenium hysterophorus in plots where members of family Amaranthaceae dominated indicated the antagonistic/allelopathic effect of amaranth members on spreading of Parthenium. The phenomenon of inhibitory or allelopathic effect has been reported in many plant species interactions including the effect of aster, Blumea lacera L. on rice and common Kharif weeds, 22-26.
Recently, the allelopathic effect of the world’s worst invasive plant Lantana camara has been revealed in terms of chemotoxicity and severe oxidative imbalance in target crop legumes 27. However, role of chromosomal rearrangements, ploidy level variations and other intrinsic biochemical mechanisms have been suspected behind aggressiveness of alien invasions, as polyploid species and favorable chromosomal rearrangements, reported in legumes like Lathyrus 28, 29, 30 may have better fitness than common native plants 31.
Furthermore, aneuploid genomes and diploid mutated genotypes showing altered morphological, biochemical and molecular make-up may acquire new strategy towards adaptations under diverse stresses 32, 33, 34, 35, 36, and thus, the origin of new invasive flora cannot be ruled out. The steep rise in population of 11 plants species in the present investigated area during the last six years has been revealed by quadrat plots. These 11 plants, therefore, were selected as an indicator of an alien invasion in the present study area, and their distribution data manifested as the ratio of interior village/forest, and roadside was presented. The four taxa of asters (Parthenium, Ageratum, Eupatorium, Chromolaena) with 5 species, assessed by this parameter, had not been used by local people in any purposes, but the two legumes, Cassia and Leucaena, were extensively utilized as medicinal (anti-diabetic) and as fuel, respectively. Similarly, Trema orientalis was mainly used as fuel.
The Lantana camara, Amaranths spinosus and Alternanthera sessilis, exhibiting aggressive nature of dominance in degraded land/forest areas, have some use by local people. Utilization of invasive plant resources for diverse economic purposes has been documented in the present inventory. It was found that the majority (Ca, 49%) of the invasive plant species were used in local health care systems, followed by food and cattle feed. Uses of leguminous plants as both food and forage by village folks have considerable significance as legumes are a cheap source of plant protein with many essential amino acids, antioxidant flavonoids and minerals 37.
A recent survey in Sikkim Himalayas (India) revealed the extraordinary potential of legumes in the formulation of diverse types of ethnic food and medicined 38. Use of fruits and flowers of the ornamental legume Sesbania grandiflora by village folks in different ailments is highly beneficial for their health, as the legume is one of the richest natural sources of vitamin A 39. Conservation of legume germplasm is essential to prevent their dwindling genetic diversity throughout the world including India.
The huge potential of under-utilized and ‘poor man’s’ legume like Lathyrus in sustainable agro-biodiversity and maintenance of soil nutrition in degraded forest areas due to its remarkable hardiness against abiotic (salinity, heavy metals, etc.) and biotic stress has been recognized in recent decade and genetic improvement programs have been undertaken 40, 41, 42, 43, 44. Identification of wild legumes as alien species in the present study assumes significance for three reasons: first, their utilization in crop improvement, second, their role/effect in alteration of legume-pollinator relationship with existing native cultivars in the invaded region, and third, as almost all parts of the study area is arsenic-contaminated, their potential to accumulate toxic metals in edible part and concomitant risk to consumers.
A recent study in these directions revealed bioaccumulation of arsenic and other heavy metals in photosynthetic part of prominent crop legumes like Phaseolus vulgaris, Lens culinaris, Cicer arietinum, and Lathyrus sativus in the lower Bengal Gangetic basin caused severe agronomic loss of yield due to alteration in antioxidant defense mechanisms and severe impairment in plant growth 45, 46, 47. Quite alarmingly, an increase in seed neurotoxin level in grass pea seeds under arsenic stress has been reported 48.
Among the 103 taxa documented in the present inventory, seven species (Eupatorium odoratum, Chromolaena odorata, Eichhornia crassipes, Lantana camara, Leucaena leucocephala, Mikania micrantha, Opuntia stricta) are enlisted as world’s 100 worst invasive species 17. The dominance of tropical American flora as the alien has been attributed to the presence of their strong allelopathic effects on native species 18, 23, 27. However, it must be noted that after the introduction, the dominance of alien flora may invite stiff competition among them which can be exploited for their better management and prevention of native species extinction.
It is also important to note that loss of native floral diversity due to alien invasion cannot be straightforward in a dynamic and functional system where increasing stress factors (both biotic and abiotic) may constrain growth and reproduction of existing native species with concomitant intro-duction (accidental or deliberate) and invasion of more hardy alien species, better utilizing the rapidly depleting soil fertility, habitat fragmentation and other adverse conditions to colonize 48, 49. A glaring and specific example of legume invasion is the spread of wild Lathyrus in diverse climatic conditions through its powerful seed dispersal mechanism from dehiscing pods, a genetic improvement of which is achieved recently to accelerate its domestication 50.
CONCLUSION: Present investigation for the first time documented compositions of alien invasive plants and their status of economic utilization by local people in and around the Uttarpara region. It is alarming to note that the number of alien species in this area constitutes a major share of floral biodiversity, of which 11 species are enlisted as most aggressive invasive plants within the study area. It is also important to note that the alien plants have been used by local people for medicinal and other diverse commercial and economic purposes, although they are posing a considerable threat to the existence of native plant species in the sanctuary. The present inventory, therefore, can be utilized as an important reference for further risk assessments and management of both alien and alien invasive flora in conservation and co-existence of both native and alien flora in this ecologically significant but fragile region of lower Indo-Gangetic basin of India.
ACKNOWLEDGEMENT: Authors are grateful to the Dr. Binod Kumar Pathak, HOD, Department of Botany and local people in the study area for giving necessary support during the entire course of the study.
AUTHORS’ CONTRIBUTIONS: All authors conducted a field study, the corresponding author designed the study and prepared the manuscript, authors one and two prepared graphs, and figures, authors 3-8 conducted the bibliographic study, and all authors read and approved the final manuscript.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Mooney HA, Mack RN, McNeely JA, Neville LE, Scjei P, and Waage JK: Invasive alien species: a new synthesis, Island Press, Washington, DC, 2005.
- Lockwood JL, Hoopes MF and Marchetti MP: Invasion ecology, Blackwell, Oxford, 2007.
- Mooney HA and Hobbs RJ: Invasive species in a changing world. Island Press, Washington DC, 2000.
- Talukdar D and Talukdar T: Allien invasive legumes and allelopathy: a case study at Gangetic West Bengal, India. International Journal of Current Research 2012: 4(4): 32-40.
- Davis MA: Biotic globalization: does competition from introduced species threaten biodiversity? Bioscience 2003; 53: 481-489.
- Singh KP, Shukla AN and Singh JS: State-level inventory of invasive alien plants, their source regions and use potential. Current Science 2010: 90: 107-114.
- Talukdar D and Talukdar T: Floral diversity and its indigenous use in the old basin (Khari) of river Atreyee at Balurghat block of Dakshin Dinajpur district, West Bengal. NeBIO 2012; 3: 26-32.
- Raghubanshi AS, Rai LC, Gaur JP and Singh JS: Invasive alien species and biodiversity in India. Current Science 2005; 88: 539-540.
- Mandal FB: The management of alien species in India. International J of Biodiver and Conserv 2011; 3: 467-73.
- Srivastava A and Singh R: Key management issues of Forest-invasive species in India. Indian Journal of Environmental Education 2009; 9: 16-24.
- Sausa R, Morais P, Dias E and Antunes C: Biological invasions and ecosystem functioning: time to merge. Biological Invasions 2011; 13: 1055-1058.
- Kriticos DJ, Sutherst RW, Brown JR, Adkins SW and Maywald GF: Climate change and the potential distribution of an invasive alien plant: Acacia nilotica ssp.indica in Australia. Journal of Applied Ecology 2003; 40: 111-124.
- Thuiller W, Richardson DM and Midgley GF: Will climate change promote alien plant invasions? Springer-Verlag, Berlin, Heidelberg, 2007: 197-211.
- Bradley BA, Oppenheimer M and Wilcove DS: Climate change and plant invasions: restoration opportunities ahead? Global Change Biology 2009; 15: 1511-1521.
- Crooks JA, Chang AL and Ruiz GM: Aquatic pollution increases the relative success of invasive species. Biological Invasions 2011; 13: 165-176.
- Lowe S, Browne S, Boudjela SM and De poorter SM: 100 of the world’s worst invasive alien species. A selection from the ‘Global Invasive Species Database’ published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union IUCN, 2000: 12.
- Huang QQ, Wu JM, Bai YY, Zhou I and Wang GX: Identifying the most noxious invasive plants in China: the role of geographical origin, life form and means of introduction. Biodiversity and Conservation 2009; 18: 305-316.
- Baider C and Vincent Florens FB: Control of invasive alien weeds averts imminent plant extinction. Biological Invasions 2011; 13: 2641-2646.
- Rao RR and Murugan R: Impact of exotic adventives weeds on native biodiversity in India: implications for conservation. In: Rai LC and Gaur JP (eds.), Invasive Alien Species and Biodiversity in India, Banaras: Banaras Hindu University, 2006: 93-109.
- Feng J and Zhu Y: Alien invasive plants in China: risk assessment and spatial patterns. Biodiversity and Conservation 2010; 19: 3489-3497.
- Diez JM, D'Antonio CM, Dukes JS, Grosholz ED, Olden JD, Sorte CJB, Blumenthal DM, Bradley BA, Early R, Ibáñez I, Jones SJ, Lawler JJ and Miller LP: Will extreme climatic events facilitate biological invasions? Frontiers in Ecology and the Environment 2012; 10: 249-257.
- Talukdar D: Selenium priming selectively ameliorates weed-induced phytotoxicity by modulating antioxidant Defense components in lentil (Lens culinaris Medik.) and grass pea (Lathyrus sativus). Annual Review & Research in Biology 2013; 3(3): 195-212.
- Chon SU and Nelson CJ: Allelopathy in compositae plants. A review. Agronomy and Sustainable Development 2010; 30: 349-358.
- Turk MA and Tawaha AM: Allelopathic effect of black mustard (Brassica nigra) on germination and growth of wild oat (A. fatua L.), Crop Protection 2003; 2: 673-677.
- Sadeghi S, Rahnavard A and Azhrafi ZY: Allelopathic effect of Helianthus annuus (sunflower) on Solanum nigrum (Black Nightshade) seed germination and growth in laboratory condition. Journal of Horticultural Science and Ornamental Plants 2010; 2: 32-37.
- Neelamegam R: Allelopathic effect of Ixora coccinea on seed germination and early seedling growth of paddy (O. sativa L.). Journal of Phytology 2011; 3: 51-55.
- Talukdar D: Allelopathic effects of Lantana camara L. on Lathyrus sativus L.: Oxidative imbalance and cytogenetic consequences. Allelopathy Journal 2013; 31(1): 71-90.
- Talukdar D: Reciprocal translocations in grass pea (Lathyrus sativus). The pattern of transmission, detection of multiple interchanges and their independence. Journal of Heredity 2010; 101: 169-176.
- Talukdar D: Cytogenetic characterization of induced autotetraploids in grass pea (Lathyrus sativus). Caryologia 2010; 63 (1): 62-72.
- Talukdar D: Meiotic consequences of selfing in grass pea (Lathyrus sativus) autotetraploids in the advanced generations: Cytogenetics of chromosomal rearrangement and detection of aneuploids. Nucleus 2012; 55(2): 73-82.
- Pandit MK, Michael, Pocock JO and Kunin WE Ploidy influences rarity and invasiveness in plants. Journal of Ecology 2011; 99(5):1108-1115.
- Talukdar D: Isolation and characterization of NaCl-tolerant mutations in two important legumes, Clitoria ternatea and Lathyrus sativus L.: Induced mutagenesis and selection by salt stress. Journal of Medicinal Plants Research 2011; 5(16): 3619-3628.
- Talukdar D: Ascorbate deficient semi-dwarf asfL1 mutant of Lathyrus sativus exhibits alterations in antioxidant defense. Biologia Plantarum 2012; 56 (4): 675-682.
- Talukdar D: The aneuploid switch: Extra-chromosomal effect on antioxidant defense through a trisomic shift in Lathyrus sativus Indian Journal of Fundamental and Applied Life Sciences 2011; 1(4): 263-273.
- Talukdar D: Dwarf mutations in grass pea (Lathyrus sativus): Origin, morphology, inheritance and linkage studies. Journal of Genetics 2009; 88 (2): 165-175.
- Talukdar D and Biswas AK: Seven different primary trisomics in grass pea (Lathyrus sativus). I Cytogenetic Characterization. Cytologia 2007; 72: 385-396.
- Dixon RA and Sumner LW: Legume natural products: understanding and manipulating complex pathways for human and animal health. Plant Physiology 2003; 131: 878-885.
- Talukdar D and Talukdar T: Traditional food legumes in Sikkim Himalayas: Preparation of foods, uses and ethnomedicinal perspectives. International Journal of Current Research 2012; 4(4): 64-73.
- Padmaja M, Sravanthi M and Hemalatha KPJ: Evaluation of antioxidant activity of two Indian medicinal plants. Journal of Phytology 2011; 3: 86-91.
- Talukdar D: Cytogenetic characterization of seven different primary tetrasomic in grass pea (Lathyrus sativus). Caryologia 2008; 61:402-410.
- Talukdar D: Recent progress on genetic analysis of novel mutants and aneuploid research in grass pea (Lathyrus sativus). African Journal of Agricultural Research 4(13): 1549-1559.
- Talukdar D: Allozyme variations in leaf esterase and root peroxidase isozymes and linkage with dwarfing genes in induced dwarf mutants of grass pea (Lathyrus sativus). International Journal of Genetics and Molecular Biology 2010; 2(6): 112-120.
- Talukdar D: Effect of arsenic-induced toxicity on morphological traits of Trigonella foenum-graecum L. and Lathyrus sativus L. during germination and early seedling growth. Current Research Journal of Biological Sciences 2011; 3(2): 116-123.
- Talukdar D: An induced glutathione-deficient mutant in grass pea (Lathyrus sativus): Modifications in plant morphology, alteration in antioxidant activities and increased sensitivity to cadmium. Bioremediation, Biodiversity and Bioavailability 2012; 6: 75-86.
- Talukdar D: Modulation of plant growth and leaf biochemical parameters in grass pea (Lathyrus sativus) and fenugreek (Trigonella foenum-graecum L.) exposed to NaCl treatments. Indian Journal of Fundamental and Applied Life Sciences 2012; 2(3): 20-28.
- Talukdar D: Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris seedlings and its amelioration by exogenous nitric oxide. Physiology and Molecular Biology of Plants 2013; 19(1): 69-79, doi: 10.1007/s12298-012-0140-8.
- Talukdar D: Bioaccumulation and transport of arsenic in different genotypes of lentil (Lens culinaris). International Journal of Pharma and Bio Sciences 2013; 4(1): (B)694-701.
- Talukdar D: Changes in neurotoxin, β-N-OXALYL- L α, β-diaminopropionic acid (β-ODAP), level in grass pea (Lathyrus sativus) genotypes under arsenic treatments. Journal of Applied Bioscience 2012; 38(2): 180-185.
- Talukdar D: Species richness and floral diversity around ‘Teesta Barrage Project’ in Jalpaiguri district of West Bengal, India with emphasis on invasive plants and indigenous uses. Biology and Medicine 2013; 5: 01-14.
- Talukdar D: Genetics of pod indehiscence in Lathyrus sativus Journal of Crop Improvement 2011; 25: 1-15.
How to cite this article:
Biswas S, Maity M, Srimany S, Chatterjee S, Karmakar T, Datta R, Patra J, Koley M and Talukdar D: Compositions, distributions and status of economic plants among invasive floras of Uttarpara, West Bengal, India. Int J Pharmacognosy 2014; 1(12): 800-09. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(12).800-09.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
10
800-809
932
2685
English
IJP
S. Biswas, M. Maity, S. Srimany, S. Chatterjee, T. Karmakar, R. Datta, J. Patra, M. Koley and D. Talukdar *
Department of Botany, R.P.M. College (University of Calcutta), Uttarpara, West Bengal, India
dibyendutalukdar9@gmail.com
26 August 2014
15 October 2014
29 November 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(12).800-09
01 December 2014