COMPARATIVE PHYTO-PHARMACOGNOSTICAL STUDY ON ACACIA ARABICA AND PROSOPIS JULIFLORA
HTML Full TextCOMPARATIVE PHYTO-PHARMACOGNOSTICAL STUDY ON ACACIA ARABICA AND PROSOPIS JULIFLORA
Sarika Nigam * 1, Vikram Singh 2, Archana Dongray 1 and Dilip Kumar Chanchal 3
College of Pharmacy 1, SRGI, Ambabai, Jhansi- 284002, Uttar Pradesh, India.
Department of Pharmacognosy 2, SRI, Datia - 475661, Madhya Pradesh, India.
Department of Pharmacognosy 3, Insitute of Pharmacy, Bundelkhand University, Jhansi - 284128, Uttar Pradesh, India.
ABSTRACT: Aim of Study: Compare the phyto-pharmacognostical study of Acacia arabica and Prosopis juliflora. Material and Methods: The ethanolic extract of on Acacia arabica and Prosopis juliflora were using physio-chemical parameters and preliminary phytochemical investigation. Results: The present study was aimed at pharma-cognostical study. Plants Acacia arabica and Prosopis juliflora were studies for pharmacognostical characteristic, namely, morphology, microscopy, physicochemical, parameters which can be of utilized in identification and authentication of plants. Methanolic extracts used for the HPTLC analysis. Several medicinal properties have been scientifically established by various workers. Conclusion: In this study, I have done the comparative pharmacognostic study on Acacia arabia and Prosopis juliflora and conclude that the ethanolic extract of Acacia arabica plays a more significant role and has more significant value than the extract of Prosopis juliflora.
| Keywords: |
Acacia Arabica, Prosopis juliflora, Phyto-Pharmacognostic
INTRODUCTION: India has a rich heritage of traditional medicine constituting with its different components like Ayurveda, Siddha and Unani and traditional health care has been flourishing in this country for many centuries. Botanicals constitute of major part of these traditional medicines. With the emerging worldwide interest, in adopting traditional practices, in the health care systems by exploiting their potential, the evaluation of the botanicals in these systems of medicine in India is utmost essential 1.
Herbalism (herbal medicine) as an alternative medical therapy is defined as the use of plants or substances derived from them, in treating disease, usually by medical herbalists without an orthodox medical qualification. Before the relatively recent application of scientific method into diagnosis and therapeutics, traditional medicines were mostly herbal 2.
Ayurvedic system understanding the knowledge of plants used for Ayurvedic preparations in relation to their use as therapeutic agents, pharmacological properties, medicinal plants being imported; medicinal plant parts being exported, endangered medicinal plants and availability of medicinal plants in different bio-geographical zones of India can be utilized in drawing strategies for rational and more scientific use of medicinal plants in a way that can be extended for future scientific investigation in different aspects 3. There were thought to be roughly 1300 species of Acacia worldwide, about 960 of them native to Australia, with the remainder spread around the tropical to warm-temperate regions of both hemispheres, including Europe, Africa, southern Asia, and the Americas.
Natural Products in Medicine: Natural products are products from various natural sources, plants, microbes and animals. They can be an entire organism (e.g. a plant, an animal or a micro-organism), a part of an organism (e.g. leaves or flowers of a plant, an isolated animal organ), an extract of an organism or part of an organism and an exudate, or pure compound (e.g. alkaloids, coumarins, flavonoids, lignans, steroids and terpenoids) isolated from plants, animals or micro-organisms.
The use of natural products, especially plants, for healing is as ancient and universal as medicine itself. Natural products have been an integral part of the ancient traditional medicine systems, e.g. Chinese, Ayurvedic and Egyptian. Even now, continuous traditions of natural product therapy exist throughout the third world, especially in the orient, where numerous minerals, animal substances and plants are still in common use. This recent resurgence of interest in plant remedies has been spurred on by several factors: 4
- The effectiveness of plant medicines.
- The preference of consumers for natural therapies, a greater interest in alternative medicines and a commonly held erroneous belief that herbal products are superior to manufactured products.
- Dissatisfaction with the results from synthetic drugs and the belief that herbal medicines might be effective in the treatment of certain diseases where conventional therapies and medicines have proven to be inadequate.
- The high cost and side effects of most modern drugs.
- Improvements in the quality, efficacy, and safety of herbal medicines with the development of science and technology.
- Patients’ belief that their physicians have not properly identified the problem; hence they feel that herbal remedies are another option.
- A movement towards self-medication.
Medicinal plants are generally known as “Chemical Goldmines” as they contain natural chemicals, which are acceptable to human and animal systems. Of the 2,50,000 higher plant species on earth, more than 80,000 are medicinal in nature.
The Red Data Book of India has 427 entries of endangered species of which 28 are considered extinct, 124 threatened, 81 vulnerable, 100 rare and 34 insufficiently known species 5.
The Origin, Scope and Practice of Pharmacognosy: The history of herbal medicines is as old as human civilization. The documents, many of which are of great antiquity, revealed that plants were used medicinally in China, India, Egypt and Greece long before the beginning of the Christian era. One of the most famous surviving remnants is Papyrus Ebers, a scroll some 60 feet long and a foot wide, dating back to the sixteenth century before Christ 6. Indians also, worked meticulously to examine and classify the herbs which they came across, into groups called Gunas. Charaka made fifty groups of ten herbs each of which, according to him, would suffice an ordinary physician’s need.
Similarly, Sushrutha arranged 760 herbs in 7 distinct sets based on some of their common properties. A large portion of the Indian population even today depends on the Indian System of Medicine - Ayurveda, ‘An ancient science of life’. The well known treaties in Ayurveda are Charaka Samhita and Sushrutha Samhita. The first pharmacist, Galen, was known to have had a number of pain relieving materials, including opium in his apothecary 7.
FIG. 1: FLOWER OF ACACIA ARABICA
FIG. 2: THORN AND LEAF OF ACACIA ARABICA
FIG. 3: PLANT OF ACACIA ARABICA
Botanical name : Acacia arabica
Hindi name : Babul, Pankikar
Family : Fabaceae
Prosopis juliflora (Sw.) DC (Mimosaceae) commonly known as mesquite is a shrub or small tree native to Mexico, South America and the Caribbean. P. juliflora probably originates from Peru; it occurs naturally in dry areas of northern South America and Central America, Mexico and southern USA. It has been introduced into many tropical areas, including northeastern Brazil, Africa, Australia, Southeast Asia and the Indian subcontinent. P. juliflora is xerophytic and is adapted to many soil types under a wide range of moisture conditions. The value of the tree lies in its exceptional tolerance of drought and marginal soils. It tolerates strongly saline soils and seasonal water logging. P. juliflora has been planted successfully on soils with acid to alkaline reaction. It is sometimes said to dry out the soil and compete with grasses, particularly in dry areas 8.
FIG. 4: FLOWER OF PROSOPIS JULIFLORA
Botanical name : Prosopis juliflora
Hindi : kabuli kikar, angarajii babul, vilayati babul
Family : Fabaceae
Chemical Constituents: Steroids, tannins, leuco-anthocyanidin and ellagic acid glycosides. A new monocyclic diketone, prosopidione, and two alkaloids, namely, juliprosinene and juliflorinine, have been isolated from the leaves 9.
Parts Used: Leaves, gum, bark, pods, flowers.
MATERIALS AND METHOD:
Materials, Instruments and Chemicals: Plant materials, glass slide, grinding mixer, hot air oven, silica crucible, ash less filter paper (Whatman no.44), petridish, stoppered conical flask, rotory flask shaker alcohol (95%), chloroform water, chloral hydrate solution, water.
Collection of Plant: The plant materials were collected from the Jhansi and Lucknow.
Authentication of Plant: The materials were authenticated at Indian Grassland and Fodder Research Institute, Jhansi, India. Sample specimens have been identified as Prosopis juliflora (SW.) DC. of the family Fabaceae .
Processing of Plant Material for Study: The materials for final study were prepared by the following procedure:
Washing: Foreign material was identified and discarded through washing.
Drying: Plant material was dried in shed to prevent decomposition of the chemical constituents.
Grinding: Material grinded till homogeneous powder was formed.
Preparation of Material:
Materials, Instruments and Chemicals: Test tubes, Soxhlet apparatus, desiccators, distillation apparatus, rotory flask shaker (Selec RC 100A), vortexer (LVM 2000), freeze dry system (Labconco), TLC chamber, sprayer, digital electronic weighing balance (OHAUS), TLC plates, china dish, beakers, conical flasks, double beam UV- Visible spectrophotometer (Thermo, UV 1), CAMAG HPTLC system consists of TLC scanner 3, application device Linomat 5, twin trough plate development chamber and winCATS software (1.3.2.0) were used. All the chemicals and reagents used were obtained from Ranbaxy Fine Chemicals Ltd. New Delhi, Fischer Inorganics and Aromatics Ltd., Madras, NICE Chemicals Ltd., Cochin and Central drug House Pvt. Ltd., (CDH), New Delhi.
Solvent: Hexane, chloroform, acetone, methanol and distilled water. In successive extraction process the powdered plant material is extracted with non polar to polar solvent i.e. hexane, chloroform, acetone, methanol, water and so on the basis of the polarity of content in the plant material will be extracted out in particular solvent like non polar in hexane and chloroform, intermediate polar in acetone or high polar in methanol and water.
Soxhlet apparatus (Hot percolation method) was used for successive extraction. Here continuous extraction of a drug or any other substance which is recommended in the monograph is done. The process consists of percolating it with suitable solvents at a temperature approximately that of the boiling point of the solvent. Any apparatus that permits the uniform percolation of the drug and the continuous flow of the vapour of the solvent around the percolator may be used.
Methodology: Assembly was arranged and thimble was prepared and placed 10 g of air dried powdered drug was extracted with hexane for 3 days, than extract solution was collected and concentrate under vacuum using Rota-vapour. Then the plant material was again collected and air dried. When completely dried it was again packed back in the thimble. Same method was repeated for chloroform, acetone, ethanol and water. Finally the dried extracts were collected in pre-weighted glass vials and post-weight for each vial was taken. Extracts for fruit of Acacia arabica Linn. was collected and finally the percentage yield was calculated for all the extracts of all the parts.
Formula Used:
10 gram air dried powder contain = X gram of extr.
100 gram air dried powder contain = 100X/10 = 10X
X = difference in weight of the vial
Phytochemical Screening:
Chemical Requirement: α-naphtol, Benedict reagent, Fehling’s A and Fehling’s B, concentrated sulphuric acid, ferric chloride, Vanillin hydro-chloride reagent, sodium hydroxide, copper sulphate, Millon’s reagent, Wagner’s reagent, Hager’s reagent. Ninhydrin, Dragandroff’s reagent etc.
Procedure:
Test for Carbohydrate:
I. Molisch’s Test: Treat the extract with few drop of alcoholic α-naphthol, add 0.2 ml of concentrated sulfuric acid slowly through side surface of test tube, purple to violet color ring appears at the junction.
II. Benedict’s Test: Treat the extract with few drop of Benedict reagent (alkaline solution containing cupric citrate complex) and boil on water bath, reddish brown ppt forms if reducing suger is present.
III. Fehling’s Test: Equal volume of fehling A (copper sulfate in distilled water) and fehling B (potassium tartarate and sodium hydroxide in distilled water) reagent are mixed along with few amount of extract, boil on water bath, brick red ppt of cuprous oxide forms, if reducing sugar are present.
IV. Caramelisation: Carbohydrate when treated with strong sulfuric acid, they undergo charring with the dehydration along with burning sugar smell.
Test for Tannin:
I. Ferric Chloride Test: Extract gives blue-green color with 5% ferric chloride solution.
II. Vanillin Hydrochloride Test: Extract when treated with few drops of Vanillin hydrochloride reagent give purple red color.
III. Alkaline Reagent Test: Extract with 5% sodium hydroxide solution give yellow to red ppt within sort time.
Test for Protein and Amino Acid:
I. Test: Extract with few ml of 5% sodium hydroxide solution and 1% copper sulphate produce pink or purple color.
II. Millon’s Test: Extract with 2 ml of Millon’s reagent (Mercuric nitrate in nitric acid containing traces of nitrous acid) white ppt appears which turns red upon gentle heating.
III. Ninhydrin Test: Amino acid and protein when boiled with 0.2% solution of ninhydrin (Indane 1, 2, 3-trion hydrate) violet color appear.
Test for Alkaloids:
I. Dragendroff’s Test (Potassium Bismuth Iodide Solution): Alkaloids give reddish brown precipitate with Dragendroff’s reagent.
II. Wagner’s Test (Solution of Iodine in Potassium Iodide): Alkaloids give reddish brown precipitate with Wagner’s reagent.
III. Hager’s Test (Saturated Solution of Picric Acid): Alkaloids give yellow color precipitate with Hager’s reagent.
IV. Mayer’s Test (Potassium Mercuric Iodide): Alkaloids give yellow colour precipitate with Mayer’s reagent.
Test for Sterols and Triterpenoids:
I. Libermann-burchard Test: Extract treated with few drops of acitic anhydride, boil and cool and concentrated sulphuric acid is added from the side of the test tube, shows brown ring at the junction of two layers and the upper layer turns green which shows the presence of sterols and formation of deep red color indicate the presence of triterpinoids.
II. Chloroform extract and acetic anhydride and conc. sulphuric acid from the side wall of the test tube upper layer turns green shows the presence of steroid.
Test for Flavonoids:
I. Shinoda Test (Magnesium Hydrochloride Ribbon Test): To the extract add few fragments of magnesium ribbon and add concentrated hydrochloric acid drop wise, pink scarlet, crimson red or occasionally green to blue color appears after few min.
II. Zinc Hydrochloride Reduction Test: To extract add a mixture of zinc dust and conc. Hydrochloric acid, its gives red color after few min.
III. Alkaline Reagent Test: To the extract add few drop of sodium hydroxide solution, formation of an intense yellow color which turns to colorless on addition of few drops of dilute acetic acid indicate the presence of flavonoid.
Test for Saponins: A portion of extract obtained dissolved in distilled water and shaken vigorously honeycomb froth persisting for 15 min indicate the presence of saponins.
Test for Resins: Extract dissolve in acetone and this solution was added to distilled water turbidity indicate the presence of resins.
Test for glycoside:
I. After complete precipitate of reducing sugar the filtrate was hydrolyzed with dilute hydrochloric acid (15%) then the same test of reducing sugar was repeated red or bright precipitate indicate the presence of glycoside/polysaccharides.
II. The alcoholic, chloroform and water extractive were treated with acetic acid, ferric chloride and 2-4 drops of concentrated sulfuric acid, formation of blue colour indicate the presence of glycoside.
TABLE 1: QUALITATIVE CHEMICAL TESTS OF CHLOROFORM EXTRACTS
| Test | A. arabica
(leaf) |
A. arabica
(bark) |
A. arabica (Twig) | P. juliflora (leaf) | P. juliflora (bark) | P. julifera
(twig) |
| Steroids | + | + | + | + | + | + |
| Triterpenoids | + | + | + | + | + | + |
| Saponin | -- | -- | -- | -- | -- | -- |
| Flavonoids | -- | -- | -- | -- | -- | -- |
| Tannin | -- | -- | -- | -- | -- | -- |
| Resin | -- | -- | -- | -- | -- | -- |
| Alkaloids | + | + | + | + | + | + |
| Glycosides | -- | -- | -- | -- | -- | -- |
| Carbohydrate | -- | -- | -- | -- | -- | -- |
| Starch | -- | -- | -- | -- | -- | -- |
| Protein & amino acids | -- | -- | -- | -- | -- | -- |
+ Present, - Absent
TABLE 2: QUALITATIVE CHEMICAL TESTS OF HEXENE EXTRACTS
| Test | A. arabica (leaf) | A. arabica (bark) | A. arabica (twig) | P. juliflora (leaf) | P. juliflora (bark) | P. juliflora
(twig) |
| Steroids | + | + | + | + | + | + |
| Triterpenoids | + | + | + | + | + | + |
| Saponin | -- | -- | -- | -- | -- | -- |
| Flavonoids | -- | -- | -- | -- | -- | -- |
| Tannin | -- | -- | -- | -- | -- | -- |
| Resin | -- | -- | -- | -- | -- | -- |
| Alkaloids | -- | -- | -- | -- | -- | -- |
| Glycosides | -- | -- | -- | -- | -- | -- |
| Carbohydrate | -- | -- | -- | -- | -- | -- |
| Reducing sugar | -- | -- | -- | -- | -- | -- |
| Protein & amino acids | -- | -- | -- | -- | -- | -- |
TABLE 3: QUALITATIVE CHEMICAL TESTS OF ETHANOLIC EXTRACTS
| Test | A. arabica
(leaf) |
A. arabica (bark) | A. arabica (twig) | P. juliflora (leaf) | P. juliflora (bark) | P. juliflora
(twig) |
| Steroids | -- | -- | -- | -- | -- | -- |
| Triterpenoids | -- | -- | -- | -- | - | - |
| Saponin | -- | - | - | - | -- | -- |
| Flavonoids | + | + | + | + | + | + |
| Tannin | + | + | + | + | + | + |
| Resin | -- | -- | -- | -- | -- | -- |
| Alkaloids | -- | -- | -- | -- | -- | -- |
| Glycosides | -- | -- | -- | -- | -- | - |
| Carbohydrate | + | -- | -- | + | -- | -- |
| Starch | -- | -- | -- | -- | -- | -- |
| Protein & amino acids | -- | -- | -- | -- | -- | -- |
TABLE 4: QUALITATIVE CHEMICAL TESTS OF WATER EXTRACTS
| Test | A. arabica
(leaf) |
A. arabica (bark) | A. arabica (twig) | P. juliflora (leaf) | P. juliflora (bark) | P. juliflora
(twig) |
| Steroids | -- | -- | -- | -- | -- | -- |
| Triterpenoids | -- | -- | -- | -- | -- | -- |
| Saponin | -- | -- | -- | -- | -- | -- |
| Flavonoids | -- | -- | -- | -- | -- | -- |
| Tannin | + | + | + | + | + | + |
| Resin | -- | -- | -- | -- | -- | -- |
| Alkloids | -- | -- | -- | -- | -- | -- |
| Glycosides | -- | -- | -- | -- | -- | -- |
| Carbohydrate | + | + | + | -- | + | + |
| Sugar | + | + | + | + | + | + |
| Protein & amino acids | -- | -- | -- | -- | -- | -- |
TABLE 5: QUALITATIVE CHEMICAL TESTS OF ACETONE EXTRACTS
| Test | A. arabica (leaf) | A. arabica (bark) | A. arabica (twig) | P. juliflora (leaf) | P. juliflora (bark) | P. juliflora
(twig) |
| Steroids | _ | - | -- | - | + | + |
| Triterpenoids | -- | - | -- | + | + | + |
| Saponin | -- | -- | -- | -- | -- | -- |
| Flavonoids | -- | -- | -- | -- | - | - |
| Tannin | + | + | + | + | + | + |
| Resin | -- | -- | - | - | - | - |
| Alkaloids | - | - | - | - | - | - |
| Glycosides | -- | - | - | - | - | -- |
| Carbohydrate | -- | - | - | - | - | - |
| Reducing sugar | - | - | - | - | -- | -- |
| Fixed oils & fats | ||||||
| Protein & amino acids | -- | -- | -- | - | - | -- |
Chromatographic Analysis:
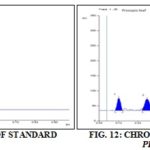
HPTLC Analysis: It was done by using 3 different reference standards namely, β-sitosterol, stigmasterol and ursolic acid. β-sitosterol, stigmasterol and ursolic acid were applied on one precoated silica gel G60 F254 Merck glass plate.
The HPTLC technique in standardization is required for-
- Quantification of marker components by area under curve.
- Determination of the accurate RF values for the marker components.
- Determination of the purity of the substance (peak purity).
- Determination of the absorption maxima of the substance.
Method:
Preparation of Methanolic Extracts: Methanolic extract of stem bark, leaf and twig of Acacia arabica and Prosopis juliflora (SW). DC. was prepared through cold percolation by using 2 g of powdered material in 100 ml of methanol.
Sample Preparation: A stock solution of methanolic extract of concentration 10 mg/ml was prepared for all the parts of Acacia arabica and Prosopis juliflora.
Standard Preparation: A stock solution of concentration 1 mg/ml was prepared for each reference standard.
Stationary Phase: Used pre-coated silica gel 60 F254 plate (E. Merck) in uniform thickness 0.2 mm.
Sample Applicator: The CAMAG Linomate-5 applicator for application of sample in the form of narrow bands, particularly analysis of mixture compound like plant extracts it is advantageous to start with compact, narrow sample application zones as they guarantee optimum resolution for a given planar chromatographic system, the CAMAG linomate-5 uses the spry-on technique for applying samples on to the chromatogram layer as narrow band this permits the application of larger sample volume than is possible with contact sample transfer, as the solvent almost completely evaporated during the process even when strongly polar solvents are used e.g. methanolic or aqueous remain contact and narrow.
When larger volume requires especially in preparative applications, a 500 µl syringe can be used instead of the standard 100 µl dosage syringe, another advantage of the linomat-5 is its self adjusting plate support. It allows the use of layers differing in thickness without readjusting the spray nozzle. This feature makes attractive for the preparative application.
Sample Application: 10 mg/ml of plant methanolic extract was prepared 10 µl of this solution was application on the plate, and 1 mg/ml standard marker solution was prepared and 10 µl of both the standard was applicated.
Solvent System: Toluene: ethyl acetate :: 8:2
Chromatography: The plate was eluted with respective mobile phase in CAMAG twin through chambers. The chamber was saturated with respective mobile phase saturation plate (E. Merck) of uniform thickness 0.2 mm was used for all the HPTLC analysis.
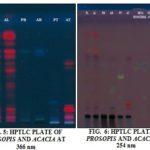
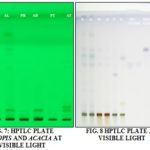
Video Documentation: The eluted plate can be analyzed under CAMAG Reproster-3 for the UV visualization at different λ value like 254 nm and 366 nm.
Scanning of Tracks: Eluted plate have different tracks of elute which are densitometrically scanned using CAMAG Scanner-3 at the respective wavelengths or at the multi wavelength for the crude extract gives area under curve for respective component present in the extract and the amount of the component will be quantify.
HPTLC Analysis:
TABLE 6: QUALITATIVE ANALYSIS
| Species and
parts |
β-sitosterol
|
Stigmasterol | Ursolic
acid |
| A. arabica (leaf) | + | - | |
| A. arabica (bark) | + | - | |
| A. arabica (twig) | + | - | |
| P. juliflora (leaf) | + | - | |
| P. juliflora (bark) | + | - | |
| P. juliflora (twig) | + |
Track 1: Prosopis leaf - 647.94 mg [sitosterol (Rf- 0.53)]:
Calculation:
Track 1: Prosopis leaf
10µl 647.94 mg
10 µl 0.6479µg
1 µl 0.6479µg ⁄ 10
1ml 0.6479mg⁄ 10
10mg/ml 0.6479mg⁄ 10
1mg/ml 0.6479mg⁄ 10 x10
Final Formula:
Calculation:
Track 2: Arabica leaf
10µl 703.22 mg
10 µl 0.7032 µg
1 µl 0.7032 µg ⁄ 10
1ml 0.7032 mg⁄ 10
10 mg/ml 0.7032 mg⁄ 10
1 mg/ml 0.7032 mg⁄ 10 ×10
Final formula:
Track 3: Prosopis bark - 549.65 mg Stigmasterol (Rf 0.52):
Calculation:
Track 3: Prosopis bark
10 µl 549.65mg
10 µl 0.5496 µg
1 µl 0.5496 µg ⁄ 10
1ml 0.5496 mg⁄ 10
10 mg/ml 0.5496 mg⁄ 10
1 mg/ml 0.5496 mg⁄ 10 x10
Final Formula:
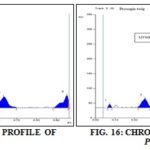
Track 4: Arabica bark - 267.87mg Stigmasterol (Rf 0.52):
Calculation:
Track 4: Arabica bark
10µl 267.87mg
10 µl 0.2678 µg
1 µl 0.2678 µg ⁄ 10
1ml 0.2678 mg⁄ 10
10 mg/ml 0.2678 mg⁄ 10
1 mg/ml 0.2678 mg⁄ 10 x10
Final Formula:
Track 5: Arabica Twig - 147.24 mg Stigmasterol (Rf 0.52):
Calculation:
Track 4: Arabica Twig
10µl 147.24mg
10 µl 0.1472 µg
1 µl 0.1472 µg ⁄ 10
1ml 0.1472 mg⁄ 10
10 mg/ml 0.1472 mg⁄ 10
1 mg/ml 0.1472 mg⁄ 10 x10
Final Formula:
Track 6: Prosopis Twig - 1.777 mg Ursolic Acid (Rf 0.43):
Calculation:
Track 6: Prosopis Twig
10µl 1.777
10 µl 0.0017 µg
1 µl 0.0017 µg ⁄ 10
1 ml 0.0017 mg⁄ 10
10 mg/ml 0.0017 mg⁄ 10
1 mg/ml 0.0017 mg⁄ 10 x10
Final formula:
TABLE 7: QUALITATIVE ANALYSIS
| Plants | Volume | mg/ml of plant extracts | % Betasitosterol | % Stigmasterol | % Urosolic acid |
| Prosopis leaf | 10µl | 647.94 ng | 0.126 | - | - |
| Arabica leaf | 10µl | 703.22 ng | 0.1230 | - | - |
| Prosopis bark | 10µl | 549.65ng | - | 0.1154 | - |
| Arabica bark | 10µl | 267.87ng | - | 0.0629 | - |
| Prosopis twig | 10µl | 1.777ng | - | - | 0.0002 |
| Arabica twig | 10µl | 147.24ng | - | 0.0228 | - |
Biological Studies:
Antioxidant Activity (Free Radical Scavenging Activity):
DPPH Radical Scavenging Assay: The effect of extract on DPPH radical was estimated using the method of Liyana-Pathirana and Shahidi.
Chemicals Requirement: 2, 2-diphenyl-1-picryl-hydrazyl (DPPH), ascorbic acid, methanol.
Standard DPPH Solution: 0.135 mM solution.
Sample Stock Solution: 0.1 mg/ml solution for all sample methanolic extract (1mg/10 ml methanol).
Methodology: A solution of 0.135 mM DPPH in methanol was prepared and 1.0 ml of this solution was mixed with 1 ml of extract in methanol containing 0.02-0.1 mg of the extract. The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min the absorbance of mixture was measured spectro-photometrically at 517 nm ascorbic acid, were used as references. The ability to scavenge DPPH radicals was calculated by the following equation:
DPPH radical scavenging activity (%) = [(Abs control - Abs sample) / (Abs control)] × 100
Where, Abs control is the absorbance of DPPH radical + methanol; Abs sample is the absorbance of DPPH radical + sample extract / reference. The same procedure was used for stem bark, leaf and twig of Acacia arabica and Prosopis juliflora.
Antioxident Activity:
TABLE 8: ABSORBANCE AT VARIOUS CONCENTRATIONS
| Concentration | 0.02 | 0.04 | 0.06 | 0.08 | 0.1 |
| Ascorbic acid | 0.153 | 0.133 | 0.114 | 0.099 | 0.116 |
| Querecitin | 0.217 | 0.182 | 0.202 | 0.211 | 0.168 |
| Acacia leaf | 0.130 | 0.140 | 0.131 | 0.127 | 0.128 |
| Acacia bark | 0.182 | 0.180 | 0.176 | 0.170 | 0.162 |
| Acacia twig | 0.150 | 0.158 | 0.153 | 0.148 | 0.162 |
| Prosopis leaf | 0.192 | 0.191 | 0.190 | 0.194 | 0.201 |
| Prosopis bark | 0.161 | 0.176 | 0.160 | 0.172 | 0.170 |
| Prosopis twig | 0.182 | 0.186 | 0.196 | 0.176 | 0.172 |
TABLE 9: PERCENTAGE OF STANDARDS AND SAMPLE
| Concentration | 0.02% | 0.04% | 0.06% | 0.08% | 0.1% |
| Ascorbic acid | 74.62% | 77.94% | 81.09% | 83.58% | 80.76% |
| Querecitin | 64.01% | 69.81% | 66.5% | 68.01% | 72.13% |
| Acacia leaf | 61.76% | 58.82% | 61.47% | 62.64% | 62.35% |
| Acacia bark | 46.4% | 47.05% | 48.2% | 50.0% | 52.3% |
| Acacia twig | 55.88% | 53.52% | 55.00% | 56.47% | 52.35% |
| Prosopis leaf | 43.52% | 43.82% | 44.11% | 42.94% | 40.885 |
| Prosopis bark | 52.64% | 48.25% | 52.9% | 48.4% | 50.0% |
| Prosopis twig | 46.47% | 45.29% | 42.35% | 48.2% | 49.45 |
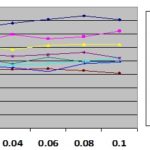
FIG. 18: DPPH % FREE RADICAL INHIBITION vs. CONCENTRATION
RESULTS:
Pharmacognostic Study: Botanical study is of prime importance in establishing quality control (identification) of herbal drugs. It may also provide asuitable criteria to differentiate the different parts used of Acacia Arabic and Prosopis juliflora (Sw). DC.
Determination of Chemical Components in Methanolic Extract of the Various Parts of Acacia arabica and Prosopis juliflora. by Using High Performance Thin Layer Chromatography (HPTLC): Result obtained from current study in HPTLC profile of methanolic extract of leaf, stem bark, twig Acacia arabica and Prosopis juliflora by CAMAG HPTLC system with wincats-3 programming software; video documentation of plates by CAMAG Reproster-3 video docu-mentation under UV 254 nm, 366 nm, and in visible light after post-derivatization with anisaldehyde sulphuric acid reagent.
Biological Studies:
Antioxidant Activity: In-vitro DPPH free radical scavenging activity of the methanolic extract of all the parts of Acacia Arabica and Prosopis juliflora (Sw.) DC. were compared with ascorbic acid and querecitin (standard used) was observed which showed that extract of Arabica leaf shows higher activity followed by bark, and twigs. At a concentration of 0.1 mg/ml the scavenging activity of the leaf reached 62.34%, while at the same concentration bark and twig have 52.3% and 52.35% activity, Prosopis leaf have minimum 40.88% activity and twig and bark have 49.4% and 50% activity.
CONCLUSION: It is concluded that given plant is Acacia arabica and Prosopis julifera, I have done the comparative pharmacognostical study between Acacia arabica and Prosopis juliflora and conclude that Acacia arabica plays more significant role and has more scientific value. phytochemical screening revealed the present of Tannin Alkaloids, steroids and Terpenoids in various extracts however most of the medicinally potential phytoconstituents where present in alcoholic and aqueous extracts, methanolic extracts used for the HPTLC analysis. Several medicinal properties have been scientifically established by various worker.
ACKNOWLEDGEMENT: The authors thankful with our deepest core of heart to Mr. Vikram Singh (Assistant Professor), for his valuable guidance.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Mukherjee PK: 2003. Exploring Botanicals in Indian System of Medicine-Regulatory Perspectives, Vol. 20, 249-264.
- Smith G: Herbs in medicine Triple Helix Autumn 04, pp. 12.
- Raghavendra MP: Alkaloid extracts of Prosopis juliflora (Sw.) DC. (Mimosaceae) against Alternaria alternate. Journal of Biopesticides 2009; 2(1): 56-59.
- Bandaranayake WM: Quality Control, Screening, Toxicity, and Regulation of Herbal Drugs, Modern Phytomedicine. Turning Medicinal Plants into Drugs. Wiley-Vch Verlag GmbH & Co. KGaA, Weinheim 2006.
- Thomas J: Medicinal and aromatic plants research in India. In UNDP 1997. Proc. Training course on Industrial Exploitation of Indigenous Medicinal and Aromatic Plants. Beijing, China 1997.
- Kokate CK: ‘Pharmacognosy’ Nirali Prakashan, Edition 37th, 2008: 105-120, 1-3.
- Agrawal SS and Paridhavi M: Herbal drug technology; Universities press (India) Private Limited, 2007: 1-5.
- Burkart A: A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). J Arn Arb 1976; 57 (3): 450–525.
- Sivakumar T: Isolation of chemical constituents from Prosopis juliflora bark and anti-inflammatory activity of its methanolic extracts. Journal of Pharmacy Research 2009; 2(3).
How to cite this article:
Nigam S, Singh V, Dongray A and Chanchal DK: Comparative phyto-pharmacognostical study on Acacia arabica and Prosopis juliflora. Int J Pharmacognosy 2018; 5(10): 646-57. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(10).646-57.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
646-657
1148
1540
English
IJP
S. Nigam *, V. Singh, A. Dongray and D. K. Chanchal
College of Pharmacy, SRGI, Ambabai, Jhansi, Uttar Pradesh, India.
sarikabbd@gmail.com
01 August 2018
26 September 2018
30 September 2018
10.13040/IJPSR.0975-8232.IJP.5(10).646-57
01 October 2018