COMPARATIVE PHYSICO CHEMICAL ANALYSIS OF VYOSHADIVATI– AN AYURVEDIC POLYHERBAL FORMULATION W.S.R TO MARKET SAMPLES
HTML Full TextCOMPARATIVE PHYSICOCHEMICAL ANALYSIS OF VYOSHADIVATI– AN AYURVEDIC POLYHERBAL FORMULATION W.S.R TO MARKET SAMPLES
Arun. M. Havinal 1, R. S. Hiremath * 2, M. B. Gundkalle 3 and V. S. Mannur 4
Department of Bhaishajya Kalpana 1, Department of Rasashastra 2, Central Research Laboratory 3, Shri B.M. Kankanwadi Ayurvedic Mahavidyalaya, Shahpur Belgaum - 590005, Karnataka, India.
KLEU College of Pharmacy 4, Belgaum - 590010, Karnataka, India.
ABSTRACT: The quality control assessment of herbal formulations is of great significance to justify their acceptability in the modern system of medicine though the drug may be therapeutically potent. Ayurvedic formulations prepared by several manufacturers are guaranteed to carry out the quality control test as per the standards mentioned in the Ayurvedic Formulary of India. Though the standards have been followed, still the variability in their results has been observed when compared between the same formulations. Vyoshadivati is one such polyherbal formulation consisted of 13 drugs and treated for ailments viz. Pinasa (coryza), Pratishaya (Rhinitis), Swarabheda (Hoarseness of voice), etc. An attempt is made here to compare Vyoshadivati prepared by GMP certified pharmacies with In house preparation. Results revealed that all the samples differ in their organoleptic, pH, and physicochemical properties. Thin Layer Chromato-graphic study showed sample B, D and E have an almost similar number of bands at the wavelength of 255 nm and 365 nm. Major difference was seen in disintegration time and hardness of sample A, i.e. hardness is 7.78 but disintegrates in 22 min whereas sample D hardness 2.63 but disintegrates in 78 min. The physicochemical data of this comparative study assists in maintaining the standard limits of Vyoshadivati.
| Keywords: |
Vyoshadivati, Polyherbal formulation, Physicochemical analysis, Thin-layer Chromatography
INTRODUCTION: Ayurveda the oldest and alternative system of medicine in the present scenario has gained its popularity and demand globally due to its effective and efficacious results witnessed in various diseases and syndromes in the recent era. Herbal drugs are the core of this system of medicine, and these drugs possess all the quality required to prevent and cure the disease which is the prime motto of Ayurveda.
The principles to standardize the drugs that were developed in the ancient period were subjective and are based on the scientific background prevailing in those days. Now they are to be viewed and answered looking towards the advancement of science and technology in the present scenario.
Hence, there is a prime need to validate Ayurvedic formulations with the aid of modern sophisticated instrumental and analytical techniques explained in the context of herbal medicine to justify the quality of the products. To meet the needs of enormous population numbers of manufacturing companies have come into existence. These manufacturers though prepare the similar formulation fails to meet the standard quality control parameters when compared.
Vyoshadivati 1 is one such polyherbal formulation explained in the Ayurvedic classics which is a combination of 13 drugs and has its efficacy over Pinasa (coryza), Pratishyaya (Rhinitis), Swarabheda (Harness of voice), Kasa (a cough), Shwasa (asthma) 1, 2, etc. most of which simulate to symptoms of upper respiratory tract infection. Based on the above rationale the present study is designed to compare Vyoshadivati prepared in house with different market samples based primary quality control parameters.
MATERIALS AND METHODS:
Pharmaceutical Part:
Raw Material Procurement:
Market Samples: Four samples of Vyoshadivati manufactured by GMP certified pharmacy were collected from the Belgaum market and given the code as Vv-A, Vv-B, Vv-C, and Vv-D.
In House Sample Preparation:
Plant Material: Vyoshadivati consists of 13 herbal ingredients. All the drugs of Vyoshadivati were procured from GMP certified KLE Ayurveda Pharmacy Khasbag Belgaum, Karnataka and were authenticated at AYUSH approved Central Research Laboratory of KLE University’s Shri B.M. Kankanwadi Ayurved Mahavidhyalaya Belgaum, Karnataka, India.
Method of Preparation:
Instruments and Equipment: Weighing machine, Analytical balance, Pulveriser, Clean cotton cloth, Steel vessel, Mask, Cap, Apron, Sieve no 85 and 120, Gas and stove.
Preparation of Churna: The Churna (powder) was prepared as per the procedure explained in Ayurvedic Formulary of India. All drugs (except Tamarindus indica Linn.) were made into a fine powder in a pulverizer. These churn are passed first through 85# mesh followed by 120# sieve individually, and then all are mixed in specified proportions to get a uniformly blended homogenous mixture.
TABLE 1: INGREDIENTS OF VYOSHADIVATI
| S. no. | Ingredients | Latin Name 3 | Part used | Quantity |
| 1. | Pippali | Piper longum Linn. | Fruit | 1part |
| 2. | Marich | Piper nigrum Linn. | Fruit | 1part |
| 3. | Shunthi | Zingiber officinale Roxb. | Rhizome | 1part |
| 4. | Chavya | Piper cheba Hunter | Stem | 1part |
| 5. | Chitrak | Plumbago zeylanica Linn. | Root | 1part |
| 6. | Jeerak | Cuminum cyminum Linn. | Fruit | 1part |
| 7. | Talisapatra | Abbes webbiana Lindl. | Leaves | 1part |
| 8. | Amlavetasa | Rhume emodi Wall. ex Meissn. | Stem | 1part |
| 9. | Tintidika | Tamarindus indica Linn. | Fruit | 1part |
| 10. | Twak | Cinnamomum zeylanica Blume. | Stembark | 1/4th part |
| 11. | Ela | Elettariac ardmomum Maton. | Seed | 1/4th part |
| 12. | Tamalapatra | Cinnamomum tamala Ness. | Leaves | 1/4th part |
| 13. | Guda(Jaggery) | - | - | 20 parts |
Preparation of Vyoshadivati: 4
Step 1: Weigh the jaggery in a specified quantity and pound it.
Step 2: 1 part of Tamarind soaked in 6parts of water for an hour later macerated and filtered through muslin cloth.
Step 3: Jaggery syrup was prepared by heating the jaggery and q.s (10 ml) water along with tamarind juice till it gets 2 thread consistency.
Step 4: Stop heating and add the homogenous mixture of churna to Jaggery syrup with continuous stirring.
Step 5: Round pills were prepared and dried in the shade.
Step 6: Vati’s are then stored in airtight container. In house, sample has given the code Vv-E
Analytical Part: To carry out physico-chemical analysis, the standard parameter has been applied as per Standards of Ayurvedic Pharmacopoeia of India. Analytical study was carried out in AYUSH approved Central Research Laboratory of Shri B. M.K. Ayurveda Mahavidyalaya Belgaum. Microbial Limit Test was carried out in Microbiology Laboratory of KLE University’s Shri B. M. Kankanwadi Ayurveda Mahavidhyalaya Belgaum, Karnataka, India. The samples had been analyzed for organoleptic characters, moisture content, extractive values, ash values 5, 6 qualitative estimation through TLC 5, physical test for tablet, i.e. hardness, disintegration and uniformity of weight 7, phytochemical analysis, and microbial limit test 8.
RESULTS AND DISCUSSION: Physicochemical analysis of Vyoshadivati market samples and in house preparation has been carried out, and the results are shown in tables. Ayurvedic formulations claimed to be made according to CCRAS guidelines are effective, but it is very difficult to maintain uniformity in formulations which is may be due to natural heterogeneity, the quality of herbal starting material obtained from wild collection shows more and more fluctuations which can be depicted from our experimental data 9.
Organoleptic Study: Organoleptic of samples reveals adequate differences observed in the presentation of the formulation and their taste, i.e. sample A and B are punched tablet indicates about the addition of some binders and are light brown whereas sample D and E are handmade round pills and are dark brown and brown. The drawback of Sample C is its irregular shape for which the analysis of physical test for a tablet doesn’t apply. This might be because as some references in classic mention the dosage form as Vataka which is a synonym for both Vatikalpana and Avaleha-kalpana.
TABLE 2: ORGANOLEPTIC CHARACTERS
| S. no. | Sample | Colour | Odor | Taste | Form |
| 1 | Vv-A | Light Brown | Characteristic | Sweet, Pungent | Punched Tablet |
| 2 | Vv-B | Light Brown | Characteristic | Pungent, Slight Bitter | Punched Tablet |
| 3 | Vv-C | Browm | Characteristic | Sweet, Pungent | Irregular Shape |
| 4 | Vv-D | Dark Brown | Characteristic | Sweet, Bitter, Pungent | Round Pills |
| 5 | Vv-E | Brown | Characteristic | Sweet, Sour, Pungent | Round Pills |
Moisture Content: Moisture content in a drug is an important tool for the stability of any formulation. If moisture is high, it provides a healthy environment for microbial growth. Sample C and E have least, and sample B has high moisture content, i.e. 12%.
Ash Value: Ash value represents the amount of non-physiological components present in the drug 10. Lesser the amount ash, less the impurities. Sample E and sample C has lesser ash value when compared to other samples i.e. 4.597% w/w and 3.53% w/w.
Extractive Values: Extractive value explains the amount of constituents that are extracted from a drug in a given solution. As the vati is administered along with water as a common anupana, maximum extraction must be observed in aqueous extract. All the samples have shown high aqueous extractive value.
pH Value: pH determines acidity or alkalinity of a drug. The pH for sample C is 5.52 whereas sample E had 4.05 and other samples are between D and E pH. Sample E is acidic compared to other samples.
TABLE 3: DETERMINATION OF MOISTURE CONTENT, ASH VALUES, AND EXTRACTIVE VALUES
| S.
no. |
Samples | Moisture content | Total
Ash |
Acid Insoluble Ash | Alcoholic Extract | Aqueous Extract | pH |
| 1 | Vv-A | 9% w/w | 10.33% w/w | 7% w/w | 23.2% w/w | 69.6% w/w | 4.50 |
| 2 | Vv-B | 9.5% w/w | 13.67% w/w | 8% w/w | 36.8% w/w | 49.6% w/w | 4.97 |
| 3 | Vv-C | 3.49% w/w | 4.597% w/w | 1.087% w/w | 17.6% w/w | 76.4% w/w | 5.52 |
| 4 | Vv-D | 12% w/w | 7.65% w/w | 2.522% w/w | 25.9% w/w | 41.4% w/w | 4.45 |
| 5 | Vv-E | 4.09% w/w | 3.53% w/w | 0.846% w/w | 34.16% w/w | 86.9% w/w | 4.05 |
Physical Characteristics for Tablets: The physical test for tablets, i.e. weight variation test where 20 tablets are randomly selected and weighed. The mean and standard deviation was calculated. Here in this study sample 1 and 2 had shown less deviation whereas samples 4 and 5 have shown a significant difference in their weight. This might be due to the pills prepared were handmade.
The disintegration time and hardness of tablet are important tools for physical stability and absorption rate. Both procedures must be directly proportional to each other. But in this study, the hardness of sample A is 7.78 kg/cm3 but disintegration time is 22 min. Whereas, hardness of sample D is 2.63 kg/cm3 and disintegration time is 78 min.
Disintegration test of both samples was done for 18 tablets. Such change affects the bioavailability of the drug to withstand in the body and show its efficacious results. The hardness of sample E is 2.1 kg/cm3 and disintegration time is 15 min.
TABLE 4: STATISTICAL ANALYSIS OF WEIGHT VARIATION TEST
| S. no. | Samples | Number of Samples | Mean | S.D. | S.E.M |
| 1 | Vv-A | 20 | 0.3000 gms | 0.007947 | 0.001777 |
| 2 | Vv-B | 20 | 0.2905 gms | 0.009987 | 0.002233 |
| 3 | Vv-D | 20 | 0.4265 gms | 0.01424 | 0.003185 |
| 4 | Vv-E | 20 | 0.5335 gms | 0.02796 | 0.006252 |
S.D – Standard deviation, S.E.M – Standard error mean
TABLE 5: DETERMINATION OF TABLET DISINTEGRATION AND HARDNESS
| S. no. | Samples | Hardness* | Disintegration** time |
| 1 | Vv-A | 7.78 kg/cm2 | 22 min |
| 2 | Vv-B | 3.8 kg/cm2 | 47 min |
| 3 | Vv-D | 2.63 kg/cm2 | 78 min |
| 4 | Vv-E | 2.1 kg/cm2 | 15 min |
* Monsanto’s Hardness Tester, **Tablet Disintegration Apparatus - Solution – Distilled water, Temperature – 39 °C, Oscillations – 30/min
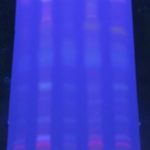
Thin Layer Chromatography: Qualitative analysis, i.e. TLC study is carried out on 60F254 pre-coated TLC plates under the solvent system toluene and ethyl acetate in the ratio 7:3 after various trial and errors. Ethanol extracts of all the samples have been taken and visualized under UV light chamber at the range of 255 nm and 365 nm. This parameter gives an idea about qualitative estimation presence of various components of drugs. Results of TLC are shown in Table 6. Sample E has shown the highest number of bands, i.e. at 255 nm 10 bands and 365nm 18 bands. When compared sample B, D, and E having an almost similar number of bands in long wavelength.
Microbial Limit Test: Microbial limit test has been carried out for all the samples and study reveals all samples were within limits as per the Indian Pharmacopeia Standard.
TABLE 6: DETERMINATION OF TLC STUDY
| S. no. | Samples | Wavelength | Rf Values |
| 1 | Vv – A | 255 nm | 0.03, 0.07, 0.16, 0.37, 0.47, 0.52, 0.58, 0.62 |
| 364 nm | 0.03, 0.07, 0.11, 0.37, 0.49, 0.55, 0.62, 0.69, 0.86, 0.96 | ||
| 2 | Vv – B | 255 nm | 0.03, 0.05, 0.1, 0.16, 0.3, 0.34, 0.49, 0.56, 0.65 |
| 364 nm | 0.02, 0.05, 0.1, 0.13, 0.24, 0.32, 0.39, 0.50, 0.54, 0.56, 0.61, 0.72, 0.77, 0.88, 0.92, 0.96 | ||
| 3 | Vv – C | 255 nm | 0.03, 0.08, 0.1, 0.15, 0.34, 0.43,, 0.50, 0.57, 0.66 |
| 364 nm | 0.03, 0.08, 0.13, 0.17, 0.20, 0.30, 0.37, 0.50, 0.54, 0.58, 0.63, 0.9 | ||
| 4 | Vv – D | 255 nm | 0.01, 0.05, 0.09, 0.16, 0.21, 0.29, 0.43, 0.52, 0.56, 0.66 |
| 364 nm | 0.01, 0.05, 0.09, 0.13, 0.21, 0.30, 0.37, 0.50, 0.53, 0.57, 0.63, 0.71, 0.8, 0.83, 0.90, 0.93, 0.96 | ||
| 5 | Vv – E | 255 nm | 0.03, 0.06, 0.10, 0.16, 0.24, 0.33, 0.43, 0.50, 0.56, 0.64 |
| 364 nm | 0.03, 0.07, 0.10, 0.13, 0.24, 0.32, 0.37, 0.50, 0.53, 0.56, 0.62, 0.70, 0.77, 0.83, 0.87, 0.9, 0.94, 0.96 |
*0.24 - The standard Rf- value of purified Piperine12
TABLE 7: MICROBIAL LIMIT TEST
| S. no. | Microbial Organism | Limit as per IP | V.V -A | V.V -B | V.V -C | V.V -D | V.V –E |
| 1 | Escherichia coli | Absent | Absent | Absent | Absent | Absent | Absent |
| 2 | Staphylococcus aureus | Absent | Absent | Absent | Absent | Absent | Absent |
| 3 | Pseudomonas aeruginosa | Absent | Absent | Absent | Absent | Absent | Absent |
| 4 | Salmonella ebony | Absent | Absent | Absent | Absent | Absent | Absent |
CONCLUSION: Vyoshadivati is a polyherbal formulation treated for the ailments pinas, swarabheda, pratishyaya, kasa, was. Being the same formulation prepared by various manufacturers yet there is a difference observed when markets samples and in house preparation are compared through standard quality control parameters as per Ayurvedic Pharmacopeia of India. Hence, it is the need of the hour that the Ayurvedic formulations are to be standardized to make them potent and therapeutically efficient.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Himasagarchandra MP: Translator Sharangadhara. Sharangadhara Samhitachapter 7. Versus 22-3. Varanasi: Chaukhamba Sanskrit Series Office; Edition 2nd, 2007: 180.
- Tripati: Indradeva Chakradattachapterkasachikitsa versus 36-7. Varanasi; Chaukhamba Sanskrit bhavan, Reprint-2010: 104.
- Sri G: Bapalal Nighantu Adarsha, uttarardha Varanasi; Chaukhambha Bharati Academy, Reprint-2005, Vol. 1 and 2.
- Himasagarchandra MP: Translator Sharangadhara. Sharangadhara Samhitachapter 7. Versus 22-3. Varanasi: Chaukhamba Sanskrit Series Office; Edition 2nd, 2007: 180.
- The Ayurvedic Pharmacopeia of India: New Delhi: Ministry of Health and Family Welfare, Government of India; Edition 1st, Part-2, Vol-1, Appendix-2, 2007: 140, 141, 195.
- The Ayurvedic Pharmacopeia of India: New Delhi: Ministry of Health and Family Welfare, Government of India; Edition 1st, Part-2, Vol-1, Appendix-3, 2007: 191.
- The Ayurvedic Pharmacopeia of India. New Delhi: Ministry of Health and Family Welfare, Government of India; Edition 1st, Part-2, Vol-3, Appendix-3 2010: 214 (3.18), 216.
- Khandelwal KR: Practical Pharmacognostic Techniques and Experiments. Pune: Nirali publications. Edition 19th, 2008: 141-152.
- Agarwal K, Bisht G, Verma S and Saini P: Pharmacieglobale International Journal of Comprehensive Pharmacy, Comparative Standardization of Polyhedral Ayurvedic Formulation: Glunorm 2012; 3(5): 55
- Quality control methods for medicinal plant materials. WHO, Geneva. AITBS. Publishers & Distributors (Regd.) Delhi. 51. 2002; 22, 28, 30
- Tanna I, Samarakoon SMS, Chandola HM and Shukla VJ: AYU Journal, Physico-chemical analysis of Herbomineral compound Mehamudgaravati- A pilot study, 2011; 32: 4. Deepthi Swapna PR, Junise V, Shibin P, Senthil S and Rajesh RS: Der pharmacia letter scholars research library, Isolation, identification and anti-mycobacterial evaluation of piperine from Piper longu, 2012; 4 (3): 863-8.
How to cite this article:
Havinal AM, Hiremath RS, Gundkalle MB and Mannur VS: Comparative physicochemical analysis of Vyoshadivati- an Ayurvedic polyherbal formulation W.S.R to market samples. Int J Pharmacognosy 2014; 1(8): 528-33. doi link: http://dx.doi.org/10.13040/ IJPSR.0975-8232.IJP.1(8).528-33.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
8
528-533
624
2080
English
IJP
A. M. Havinal*, R. S. Hiremath, M. B. Gundkalle and V. S. Mannur
Department of Bhaishajya Kalpana *, Shri B.M. Kankanwadi Ayurvedic Mahavidyalaya Shahpur Belgaum, Karnataka, India
rasamruta@yahoo.co.uk
08 February 2014
08 May 2014
28 June 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(8).528-33
01 August 2014