CHROMATOGRAPHIC ANALYSIS AND ANTIOXIDANT ACTIVITIES OF THE AERIAL PART ESSENTIAL OIL OF SOLENOSTEMON MONOSTACHYUS (P. BEAUV)
HTML Full TextCHROMATOGRAPHIC ANALYSIS AND ANTIOXIDANT ACTIVITIES OF THE AERIAL PART ESSENTIAL OIL OF SOLENOSTEMON MONOSTACHYUS (P. BEAUV)
Samuel Ehiabhi Okhale *, Chinyere Imoisi, Samuel Sunday Ode, Josiah Gana James and Akeem Lateef
Department of Medicinal Plant Research & Traditional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, P.M.B. 21 Garki, Abuja, Nigeria.
ABSTRACT: The present study aimed to investigate the essential oil of Solenostemon monostachyus aerial part extract and its antioxidant and phytochemical potency. The essential oil of S. monostachyus aerial part was obtained by hydrodistillation using Clevenger-type apparatus, and the aqueous extract by infusion. Gas chromatography-mass spectrometry analysis of the essential oil gave twenty-two compounds as the major constituents with their retention factor. A total of forty-three compounds were identified of which limonene (55.40 %) and neral (10.39%) were found as major compounds followed by trans-verbenol (6.43%) and decanal (3.25%). The aqueous extract's phytochemical analysis revealed terpenes, alkaloids, sterols, tannins, reducing sugar, flavonoids, saponins, and anthraquinones. HPLC analysis gave sixteen peaks, including tannins, caffeic acid, rutin, ferulic acid, morin, and apigenin. S. monostachyus exhibited antioxidant activity from the first dilution at 20 mg/mL up to the ninth dilution at 0.08 mg/mL in 2-fold dilutions signifying the presence of antioxidant compounds at those concentrations. Therefore, the results showed that the aqueous and hydroalcoholic extract of Solenostemon monostachyus exhibited antioxidant activity.
Keywords: Solenostemon monostachyus, DPPH, Antioxidant, Antimicrobial, Essential oil, Phytochemicals
INTRODUCTION: Medicinal plants have been used as sources of drugs by mankind for several years. In fact, ancient man depended on plants for his needs of treatment, prevention, and other medicaments, thus utilizing plants as drugs for millennia. For the past 3000 years, many plants have been used in health care practices, such as Traditional Medicine in China, India and Africa, most of which contain therapeutic values that Western standards have ascertained.
Furthermore, several other plants have been employed for centuries by several cultures, which are less likely to be proven by Western standards. Medicinal plants, however, remain a major source of novel drug discovery and development and have contributed greatly to human health.
Nature’s pharmacy has given humans an endless supply of plants enriched with various secondary metabolites for man to harness. Plants have provided an endless array of chemicals for man since the beginning of man’s existence. Several oxidation reactions take place in the biological system resulting in oxidative stress; one of such is the univalent reduction of oxygen which results in the conversion of a certain percentage of inhaled oxygen into reactive species or free radicals called reactive oxygen species (ROS) 1.
Antioxidants are also called free radical scavengers and are compounds whose presence, even at low concentrations, possess the ability to prevent oxidation of cell constituents like lipid, carbohydrates, DNA and protein; they act via a number of processes such as by preventing the formation of free radicals through preventing phagocyte activation; through binding of transition metal ions and prevention of hydroxyl formation and via repair of damage as in tocopherol which repairs peroxyl radicals thereby halting lipid peroxidation and preventing decomposition of lipid hydroperoxide 1.
Solenostemon monostachyus is an annual, aromatic and succulent weed belonging to the family Lamiaceae and is spread widely in West and Central Africa. It is found in rocky savannah and in anthropogenic habitats. It can grow up to 100 cm high. The plant's aerial part is traditionally used in Nigeria as a decoction to manage malaria 2.
It possesses anti-inflammatory and antinociceptive activity 3, antisickling effects 4, diuretic 5, antimicrobial activities 6, antihypertensive 7 and antiulcer activities 8. S. monostachyus is commonly referred to as monkey’s potato and has great ability to scavenge hydroxyl and hydrogen peroxide free radicals and cause in-vitro reduction of ferric ions, an activity which suggests its antioxidant potential 9.
The leaves of S. monostachyus is used as a vegetable, the leaf sap is used for treating rheumatism, heamaturia, menstrual pain, infertility in female, snake bites, fever, eye problems, foot infections, headache and convulsion. The root has been used in the treatment of onchocerciasis 10. It also possesses hypolipidaemic and antidiabetic effect 11. The plant is used to treat skin infections 12, 13. Herbalists in Abidjan uses S. monostachyus mixed with powder kaolin (white) to treat female sterility 14. Phytochemical screening reveals secondary metabolites such as polyphenols, flavonoids and coumarins 15, tannins, saponnins, alkaloids, phytates, reducing sugars, flavonoids, carbohydrate and anthraquinone 16. The leaf essential oil of S. monostachyus has been reported to contain; β-pinene, oct-1-en-3-ol, β-caryophyllene, octan-3-ol and (E, E)-α-farnesene 3.
MATERIALS AND METHODS:
Chemicals and Reagents: Unless otherwise stated all chemicals and reagents were of analytical grade and purchased from Sigma Aldrich (Germany).
Collection and Identification of Plant Sample: The plant material was collected from Idu industrial area, Abuja, Nigeria; it was identified and authenticated by a Taxonomist at the herbarium of National Institute for Pharmaceutical Research and Development (NIPRD), Abuja, Nigeria, where the voucher specimen (NIPRD/H/6817) was deposited.
Preparation of plant extracts: The aerial part of S. monostachyus was crushed using mortar and pestles; the crushed sample was macerated in 70% ethanol for 24 hours, allowed to stand overnight, and filtered using the burchner filter. The filtrate was dried over a water bath. The yield was determined and dried extract was stored in airtight container until use. A second extraction was carried by which the aerial part of S. monostachyus was cut using scissor and hydro distillation was carried out to extract oil and to be used for antimicrobial analysis. The hot water extract was filtered and dried over water bath. The dried extract was stored in a container until use. The third extraction carried out was infusion extraction. The aerial part of the plant was dried for three weeks and then crushed, 25g of the crushed plant was poured into jamb bottle and 500 mL of boiled water was added and allowed to stand for 24 hours, after which it was filtered and the filtrate was concentrated over a water bath. This process was carried out twice to get enough extract to be used for further analysis.
FIG. 1: IMAGE OF SOLENOSTEMON MONOSTACHYUS IN ITS NATURAL HABITAT
Phytochemical Analysis: Phytochemical screening of Solenostemon monostachyus was carried out to determine the presence or absence of various phytochemicals, the following were the methods:
Test for Saponins:
Froth Test: small quantity of the crude extract was added to 10 mL of distilled water in a test tube. The test tube was stoppered and shaken vigorously foe about 30 seconds. It was allowed to stand for half an hour. Persistent honeycomb froth is indicative for the presence of saponins.
Fehling Solution Test: To 2 ml of the crude plant extract was added to an equal amount of Fehling’s solution A and B. A bluish-green precipitate shows the presence of saponin glycosides.
Test for Tannins: Ferric chloride test: The boiled water extract was diluted with distilled water in a ratio 1:4, and a few drops of 10% ferric chloride solution were added. A green or blue-black color indicates the presence of tannins.
Test for Terpenes and Sterols:
Liebermann-Burchard Test: The crude extracts were added to 50 mL of ethanol (95%) and evaporated to dryness. The residue was dissolved in 10 mL of anhydrous chloroform and mixed with 1 mL acetic anhydride, followed by adding 1 mL of concentrated sulphuric acid down the wall of the test tube to form a lower layer.
The formation of reddish-violet color at the junction of the liquids and green color in the chloroform layer indicates the presence of terpenes. Salkowski’s test: The crude extracts were added to 50 mL of ethanol (95%) and evaporated to dryness. The residue was dissolved in 10 mL of anhydrous chloroform and mixed with 2 mL of concentrated sulphuric acid carefully so that the acid forms a lower layer. A reddish-brown color at the interface indicates the presence of a steroid.
Test for Flavonoids:
Sodium Hydroxide Test: The sample was completely extracted with acetone. Filter and evaporate the extract to dryness to obtain the residue (detanned). 5 mL of 10% sodium hydroxide was added to an equal volume of the detanned water extract. A yellow solution indicates the presence of flavonoids.
Ferric Chloride Test: The sample was completely extracted with acetone. Filter and evaporate the extract to dryness to obtain the residue (detanned). 2 mL of the detanned water extract was diluted with distilled water in a ratio of 1:4 and a few drops 10% ferric chloride solution were added. A green solution indicates the presence of phenolic nucleus.
Test for Alkaloids: The crude extract was prepared by macerating in 50 mL of ethanol. The extract was evaporated to dryness. The residue was mixed with 10 mL of 1% aqueous hydrochloric acid. 1 mL of the prepared extracts was treated with a few drops of the following reagent: Meyer’s reagent forms cream precipitate. Dragendorff’s reagent forms an orange/reddish brown precipitate. Hager’s reagent forms a yellow precipitate. Turbidity or precipitation with all of these reagents is indicative of the presence of alkaloids in the extract.
Test for Sugar: Fehling’s solution test for free reducing sugars: 2 mL of the water extract was added to 5 mL of the mixture of equal volume of Fehling solutions A and B and boiled on a water bath for 2 minutes. A brick red color indicates the presence of reducing sugar.
Fehling’s test for Combined Reducing Sugars: 1 mL of the water extract was hydrolyzed by boiling with 5 mL of dilute hydrochloric acid and was neutralized with sodium hydroxide solution. The Fehling’s test was repeated. A blue color indicates the presence of combined sugar.
Test for Carbohydrate (Molisch test): Few drops of Molisch’s reagent were added to 1 mL of the extract and 0.5 mL of conc. Sulphuric acid was also added and allowed to form a layer. Formations of purple rings at the interface of the liquid indicated the presence of carbohydrates.
Test for Anthraquinone: Few drops of potassium hydroxide were added to 1mL of the extract. The formation of pink color indicates the presence of anthraquinone.
High-Performance Liquid Chromatography Analysis (HPLC): The chromatographic system includes Shimadzu HPLC system consisting of Ultra- Fast LC-20AB prominence equipped with SIL- 20AC autosampler; DGU-20A3 degasser; SPD-M20A UV diode array detector (UV-DAD, wavelength of 190-800 nm); column oven CTO-20AC, system controller CBM- 20Alite and Windows LC solution software (Shimadzu Corporation, Kyoto Japan); column, VP-ODS 5µm and dimensions (150 x 4.6 mm). The chromatographic conditions included mobile phase solvent A: 0.2% v/v formic acid and solvent B: acetonitrile; mode: isocratic; flow rate 0.6 ml/min; injection volume 5µl of 20 mg/ml solution of HYP in water; detection was at UV 254 nm wavelength. Reference standards, rutin, quercetin, caffeic acid, ferulic acid, and apigenin (Fluka, Germany) 50 µg/ml in methanol were analyzed separately under the same condition as the extract (HYP). The HPLC operating conditions were programmed to give the following: solvent B: 20% and column oven temperature 40 oC. The total run time was 40 minutes.
Essential Oil Extraction: The fresh bulbs (500 g) were chopped into small pieces and hydrodistilled using a Clevenger-type apparatus. The extraction was carried out for 4h. The colorless oil obtained was dried over anhydrous sodium sulphate. The oil was filtered through 0.22 microns filter and kept at 4oC in sealed vials in dark until analysis.
Gas Chromatography-Mass Spectral Analysis: The essential oil was analyzed by GC-MS according to the method described by 17 using Shimadzu QP-2010 GC with QP-2010 mass selective detector [MSD, operated in the EI mode (electron energy = 70 eV), scan range = 45-400 amu, and scan rate = 3.99 scans/sec], and Shimadzu GCMS solution data system. The GC column was HP-5MS fused silica capillary with a (5% phenyl)- polymethylsiloxane stationary phase, length 30m, internal diameter 0.25 mm and film thickness 0.25 μm. The carrier gas was helium with flow rate of 1.61 ml/min. The program used for GC oven temperature was isothermal at 60oC, followed by 60-180oC at a rate of 10oC/min, then held at 180oC for 2 minutes; followed by 180-280oC at a rate of 15oC/min, then again held at 280oC for 4 minutes. The injection port temperature was 250oC. The ionization of sample components was performed in the E.I. mode (70eV). Injector temperature was 250oC while detector temperature was 280oC. Helium was used as carrier gas at a 1.61 ml/min flow rate. 1.0µl of diluted sample (1/100 in hexane, v/v) was injected using autosampler and in the split mode. Split ratio was 10:90.
Antioxidant Assay: The method of 18 was used with some modifications. A two-fold dilution of 20 mg/mL of each extract with 50µL of distilled water in a 96-well micro-dilution plate was performed. Thereafter, 50 µL of 0.2 mg/mL of 3-(4,5-dimethylthiazole - 2 - yl) - 2, 5-diphenyltetrazolium bromide (MTT) was added to every well and the plate was incubated for one week at 37oC after which the result was read. Distilled water was used as negative control and ascorbic acid was used as standard drug. Formation of bluish colouration/ precipitate was indicative of antioxidant activity. The lowest concentration of the sample at which the presence of antioxidant is detected was recorded as the minimum inhibitory concentration. This is the highest dilution at which the formation of bluish colouration disappears. The experiment was done in triplicate.
Thin Layer Chromatography Analysis of Antioxidant: The antioxidant effects of the extracts were analyzed using TLC, followed by 2,2-diphenyl -1-1-picrylhydrazy (DPPH) technique. About 100µm of the extract was loaded on the TLC plate (mark 10×10cm2).
The TLC plate was developed in hexane and ethyl acetate in the ratio 3:2 to separate the various constituents of the extracts. The developed plate was air dried and observed under visible and UV light (254 nm and 366 nm). Various separation spots were noted and their RF values calculated. After this examination; 0.05% of DPPH solution in methanol were sprayed on the surface of developed TLC plate and incubated for 10 minutes at room temperature; the active antioxidant Solenostemon monostachyus constituents were detected as yellowish white spot produced by bleaching of DPPH by resolved bands on TLC plate. After visual comparison with the intensity of bleached color of the TLC band of positive standard, the antioxidant strength of Solenostemon monostachyus constituents were tentatively categorized as strong and weak. All detected active antioxidant constituents were noted according to their RF values. Ascorbic acid and gallic acid were used as positive control and blank TLC plate was taken as negative control 19.
RESULTS AND DISCUSSION:
Phytochemical Analysis: Qualitative phyto-chemical analysis of the aqueous extracts of Solenostemon monostachyus confirmed the presence of secondary metabolites such as saponins, tannins, flavonoids, reducing sugar, antraquinones, alkaloids, terpenes and sterols as shown in Table 1.
TABLE 1: QUALITATIVE PHYTOCHEMICAL ANALYSIS OF THE HYDROSOL OF SOLENOSTEMON MONOSTACHYUS
| Secondary Metabolites | Test | Observation | Inference |
| Saponins | Froth test
Fehling’s test |
Persistent Honeycomb
Persistent Honeycomb |
+ve
+ve |
| Alkaloids
Tannins |
Mayer’s test
Dragendorff’s Hager’s 10% Tannic acid Wagner’s Ferric chloride |
Creamy precipitate
Orange precipitate Yellow precipitate No precipitate No precipitate Greenish ppt |
+ve
+ve +ve +ve +ve +ve |
| Flavonoids | Sodium Hydroxide
Ferric chloride |
Yellow solution
Greenish precipitate |
+ve
+ve |
| Reducing Sugar | Fehling’s test for reducing sugar
Fehling’s test for combined reducing sugar |
Brick red color
Blue color |
+ve
+ve |
| Terpenes | Liebermanna-Burchard test | A reddish brown-violet color at the interface | +ve |
| Sterols | Salkowski’s test | A reddish-brown coloration at the interface | +ve |
| Carbohydrate | Molish reagent | Purple ring | +ve |
| Anthraquinones | Potassium hydroxide | Pink color | +ve |
The phytochemical analysis of S. monostachyus also revealed the presence of the leaf essential oil such as β-pinene, oct-1-en-3-ol, β-caryophyllene, octan-3-ol and (E, E)-α-farnesene 3. Alkaloids are naturally occurring chemical compounds containing basic nitrogen atoms and are produced by a large variety of organisms including bacteria, fungi, plants, and animals. Many alkaloids are toxic and often have a pharmacological effect, which makes them to be used as medications and recreational drugs. Some alkaloids have a bitter taste.
Flavonoids are derived from 2-phenylchromen-4-one (2-phenyl-1-4-benzopyrone) and are commonly known for their antioxidant activities. Flavonoids, which are widely distributed in plants, fulfill many functions including producing yellow, red or blue pigmentation in flowers and protection from attacks by microbes and insects. Compared to other active plant compounds, they are low in toxicity 15. Flavonoids are referred to as nature’s biological response modifiers because of their inherent ability to modify the body’s reaction to allergens, viruses and carcinogens 15. They show anti-allergic, anti-inflammatory, antimicrobial and anticancer activities. Flavonoids exhibit potent antioxidative and free radical scavenging activities. Tannins have antioxidant properties; they are astringent and excessive amounts could damage the mucosa lining of the digestive tract. Tannins have shown potential antiviral, antibacterial, and antiparasitic effects 16. Saponins are being promoted commercially as dietary supplements and nutriceuticals. There is evidence of the presence of saponins in traditional medicine preparations where oral administrations might be expected to lead to glycoside hydrolysis to aglycone (sapogenin) and glycone component 16.
Saponins are the glycosides of 27-carbon atom steroids or 30-carbon atom triterpenes in plants. They are found in various plant parts; leaves, stems, roots, bulbs, flowers, and fruits. They are characterized by their bitter taste and ability to haemolyze red blood cells 20. They are used medically as expectorant, emetic and for treating excessive salivation, epilepsy, and migraines 21. They are used in Ayurvedic medicine to treat eczema, psoriasis and remove freckles. Saponins are believed to be useful in the human diet for controlling cholesterol. Digitalis-type saponins strengthen the heart muscle causing the heart to pump more efficiently. Saponins also inhibit cancer tumor growth in animals, particularly, lung and blood cancers, without killing normal cells. They are the plant’s immune system acting as an antibiotic to protect the plant against microbes 22.
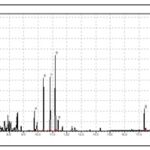
Antioxidant Activity of S. monostachyus: The antioxidant activity of S. monostachyus was performed employing MTT and 96-well microdilution technique. The concentration of the extract ranged from 20 mg/mL to 0.01mg/mL, and the concentration of the reference ascorbic acid ranged from 0.2 mg/mL to 0.0001 mg/ml. The bluish formazan formed due to the antioxidant activity of S. monostachyus was observed from the first dilution at 20 mg/ml up to the ninth dilution at 0.08 mg/mL in 2-fold dilutions signifying the presence of antioxidant compounds at those concentrations. The ascorbic acid was active from 0.2 mg/mL to 0.013 mg/ml. Therefore, the antioxidant properties of S. monostachyus could play a useful role in food preservation and the prevention of oxidative damage associated with many diseases 9. The chromatogram showed 22 chemical components, as shown in Table 2.
FIG. 2: GC–MS CHROMATOGRAM OF SOLENOSTEMON MONOSTACHYUS ESSENTIAL OIL ANALYZED
TABLE 2: CHEMICAL CONSTITUENTS OF SOLENOSTEMON MONOSTACHYUS ESSENTIAL OIL
| Peak # | Name | RI | % Composition |
| 1 | 1-Ethyl-2-methylbenzene | 973 | 3.73 1-Ethyl-2-methylbenzene is a natural product found in Gossypium hirsutum, Lavandula angustifolia and other organisms |
| 2 | 1,2,3-Trimethylbenzene | 998 | 1.65 1,2,3-Trimethylbenzene is a natural product found in Ferulago nodosa, Vitis vinifera and other organisms |
| 3 | 1-Octen-3-ol | 1009 | 2.00 1-Octen-3-OL is a natural product found in Nepeta nepetella, Origanum syriacum and other organisms |
| 4 | Mesitylene | 1013 | 6.75 Mesitylene is a natural product found in Lepidium meyenii and Ferulago nodosa |
| 5 | o-Cymene | 1025 | 5.39 O-Cymene is a natural product found in Piper nigrum, Curcuma aromatica and other organisms |
| 6 | 1-Methyl-3-propylbenzene | 1050 | 3.05 |
| 7 | 1-Methylindane | 1085 | 2.16 |
| 8 | 3,7-Dimethyldecane | 1126 | 2.62 |
| 9 | Isodurene | 1131 | 3.86 |
| 10 | Caryophyllene oxide | 1580 | 6.60 |
| 11 | Sabinol | 1140 | 1.38
Sabinol is a natural product found in Artemisia kaschgarica |
| 12 | Dodecane | 1200 | 8.59 Dodecane is a natural product found in Erucaria microcarpa, Synechocystis, |
| 13 | α-Cubebene | 1338 | 2.08 |
| 14 | β-Caryophyllene | 1415 | 6.18 |
| 15 | α-Caryophyllene | 1448 | 6.14 |
| 16 | γ-Muurolene | 1478 | 9.57 |
| 17 | α-Farnesene | 1498 | 1.18 |
| 18 | Hexadecanoic acid | 1983 | 2.26 |
| 19 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 2118 | 12.04 |
| 20 | Phytol | 2123 | 3.99 |
RI: Retention index
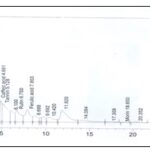
High-Performance Liquid Chromatography Analysis (HPLC): A total of nine peaks were detected from the HPLC spectrum with retention times of 2.73, 3.14, 3.64, 4.16, 5.22, 5.74, 7.76, 9.02 and 12.83 minutes as shown in Fig. 3, caffeic acid and rutin appeared at retention times of 5.22 and 7.76 minutes respectively.
The HPLC chromatogram of S. monostachyus extracts showed the presence of gallic acid, caffeic acid, ferulic acid, tannin, apigenin, and rutin, which are reputable bioactive phenolic acids and flavonoids with strong antioxidant properties, amongst others 23.
FIG. 3: HPLC PROFILE OF THE HYDROSOL OF SOLENOSTEMON MONOSTACHYUS
CONCLUSION: This study showed that the aerial part of Solenostemon monostachyus contains a good number of phytochemicals, essential oil, antioxidant, and antimicrobial activities. Also, the toxicity test shows that it is very safe to be used as herbal medicine to cure several diseases. From the results, it can be concluded that the essential oil of Solenostemon monostachyus aerial part and aqueous extracts possess antimicrobial and antioxidant activity, which justifies using Solenostemon monostachyus leaf in folklore.
ACKNOWLEDGEMENTS: The authors wish to thank the Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, Abuja, Nigeria, for providing technical support for this study.
CONFLICTS OF INTEREST: We declare that we have no conflict of interest.
REFERENCES:
- Gupta VK and Sharma SK: Plants as natural antioxidants. Natural Product Radiance 2006; 5(4): 326-334.
- Adebayo JO and Krettli AU: Potential antimalarials from Nigerian plants. A Review J Ethnopharmacol 2011; 133: 289–302.
- Okokon FJ, Koofresh D and Lucky LN: Anti-inflammatory and Antinociceptive activities of Solenostemon monostachyus aerial part extract in mice. Avicenna Journal of Phytomedicine (AJP) 2016; 6(3): 284-294.
- Afolabi SI, Iyanuoluwa OO, Oluwabukunmi DF, Priscilla IU, Damilola OO, Tolulope BA, Alaba OA and Bosede TA: Solenostemon monostachyus, Ipomoea involucrataand Carica papaya seed oil versus Glutathione, or Vernonia amygdalina: Methanolic extracts of novel plants for the management of sickle cell anemia disease. BMC Complementary and Alternative Medicine 2012; 12: 262.
- Koffi N, Marie Solange T, Emma AA and Noel ZG: Ethnobotanical study of plants used to reat arterial hypertension in traditional medicine, by abbey and Krobou population of Agboville (Cote d’ivoire). Eur J Sci Res 2009; 35: 85-98.
- Ekundayo EO and Ezeogu LI: Evaluation of antimicrobial activities of extracts of five plants used in traditional medicine in Nigeria. Intern J Trop Med 2006; 1: 93-96.
- Fidele KZ, Andre KB, Yao DJ and Michel OA: Action of hydroethanolic leaves extract of Solenostemon monostachyus (Lamiaceae) on cardiovascular system of mammalians: blood pressure lowering effects. Intl J Pharm Biol Sci 2012; 2: 310-320.
- Amazu UL, Bassey SA and Okokon EJ: Antiulcerogenic activity of Solenostemon monostachyus. The Journal of Phytopharmacology 2015; 4(2): 97-101.
- Okoko T and Ere D: Antioxidant activities of Solenostemon monostachyus leaf extract using in-vitro Sci Res Ess 2012; 7(6): 621-626.
- Lemmens RHMJ: Solenostemon monostachyus, (PROTA) plant use. Journal of the Linnean Society-Botany 2004; 58(372): 231-283.
- Okokon J, koofresh AD and Bala AA: Antipyretic and antimalaria activitites of Solenostemon monostachyus. Pharmbiol 2015; 54(4): 648-653.
- Djah FM, Danho FRN and Kouakou LK: Medicinal plants and traditional healing practices in ehotile people, around the aby lagoon (eastern littoral of coted’ivoire). Journal of ethnobiology and ethnomedicine 2015; 11(21): 1-18.
- MacDonald I, Joseph OE and Oghale OU: Ethnomedicinal Plants Used by the Idoma People- Benue State, Nigeria. American Journal of Ethnomedicine 2014; 1(1): 72-88.
- Mamadou K, Djenamba BK, Henry DK, Laurent AA, Jean–claude Y, Therese DP and Francoise H: Pharmacovigilance of Medicinal plants: Contribution of the Herbalists in Abidjan. International Journals of Phytopharmacology 2015; 6(2): 66-75.
- Datte JY, Kpahe F and Offoumou AM: Acute toxicity and antioxidant activity of hydroethanolic extract of Solenostemon monostachyus P. Beauv. Leaves. J Compl Integr Med 2010; 7: 45.
- Obichi, EA, Monago CC and Belonwu DC: Nutritional Qualities and phytochemical compositions of Solenostemon monostachyus (family Lamiaceae). Journals of Environment and Earth Science 2015; 5(3): 105-111.
- Okhale SE, Ugbabe GE, Oladosu PO, Ibrahim JA, Egharevba HO, Kunle OF, Elisha EP, Chibuike AJ and Ettah UO: Chemical Constituents and antimicrobial activity of the leaf essential oil of Ixora coccina L (Rubaiceae) collected from north central Nigeria. International Journal of Bioassays 2018; 7(5): 5630-5637.
- Muraina IA, Suleiman MM and Eloff JN: Can MTT be used to quantify the antioxidant activity of plant extracts. Phytomedicine 2009; 16: 665–668.
- Climpoiu C: Analysis of some natural antioxidants by TLC and High-performance thin layer chromatography. J Liq Chrom Rel technol 2006; 29: 1125-1142.
- Asanga E, Dennis A, Udosen EO and Uboh FE: Evaluation of the effect of ethanolic leaf extract of monostachyus on Blood glucose and liver enzymes in STZ induced diabetic rats. European Scientific Journal 2015; 11(12): 161-170.
- Chukwura EI and Iheukw E: Determination of synergistic effect and Activity index of Solenostemon monostachyus and Ocimum gratissimum on selected Journal of Scientific and Industrial Research 2013; 72: 577-580.
- Adesina AO, Ladoja F and Murtala AA: Toxicological profile of hydroethanolic extract of the leaf of Solenostemon monostachyus (p. beauv) (Lamiaceae) in rats. Advancement in Medicinal Plant Research 2015; 3(3): 126-136.
- Sulayman A and Saad T: Antimicrobial and antioxidant activity of Breynia disticha and Vernonia elaeagnifolia. J App Pharm 2015; 7(3): 178-182.
How to cite this article:
Okhale SE, Imoisi C, Ode SS, James JG and Lateef A: Chromatographic analysis and antioxidant activities of the aerial part essential oil of Solenostemon monostachyus (P. beauv). Int J Pharmacognosy 2023; 10(2): 236-43. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.10(2).236-43.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
236-243
866 KB
774
English
IJP
Samuel Ehiabhi Okhale *, Chinyere Imoisi, Samuel Sunday Ode, Josiah Gana James and Akeem Lateef
Department of Medicinal Plant Research & Traditional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, P.M.B. 21 Garki, Abuja, Nigeria.
samuelokhale@gmail.com
05 January 2023
15 February 2023
27 February 2023
10.13040/IJPSR.0975-8232.IJP.10(2).236-43
28 February 2023