CHEMICAL CONSTITUENTS FROM THE AERIAL PARTS OF LANTANA INDICA ROXB.
HTML Full TextCHEMICAL CONSTITUENTS FROM THE AERIAL PARTS OF LANTANA INDICA ROXB.
Mohammed Ali * 1, Mohammad Jameel 1, 2, Showkat R. Mir 1 and Shahnaz Sultana 1, 3
Phytochemistry Research Laboratory 1, Faculty of Pharmacy, Jamia Hamdard, P.O. Hamdard Nagar, Delhi - 110062, New Delhi, India.
Regional Research Institute of Unani Medicine 2, CCRUM, Ministry of AYUSH, Government of India, Aligarh - 202001, Uttar Pradesh, India.
College of Pharmacy 3, Jazan University, Jazan, Saudi Arabia.
ABSTRACT: Lantana indica Roxb. (Verbenaceae) is grown as an ornamental plant in tropical and subtropical regions of the world. The plant is used to treat asthma, abdominal disorders, bilious fever, cancer, catarrhal infections, chicken pox, eczema, hypertension, malaria, measles, swelling, rheumatism, tetanus, and ulcers. Phytochemical investigation of a methanolic extract of the leaves led to isolating three new chemical constituents characterized n-heneitriacont-4-en-16β, 17β, 18β-triol (n-heneitriacontenol, 3), 6, 4'- dimethoxy- 7, 3'- dihydroxy flavone- 7- O- β-D-glucopyranoside (lantanaflavone 7-O-β-D-glucoside, 6), urs-12-en-3β-ol-28-oic acid 3β-D-glucopyranosyl-6´-octadecanoate (ursolic acid 3-O-β-D-glucosyl-6'-oleate, 8) along with five known compounds characterized as n- hexadecanyl oleate (1), n-octadecanyl oleate (2), oleanolic acid (4), ursolic acid (5) and oleanolic acid 3-O-β-D-glucopyranoside (7). The structures of all the isolated phytoconstituents have been established on the basis of spectral data analysis and chemical reactions.
| Keywords: |
Lantana indica Roxb, Leaves, Chemical constituents, Isolation, Structure elucidation
INTRODUCTION: Lantana indica Roxb., syn. L. Collina Decne, L. latifolia Tausch (Verbenaceae), known as west Indian lantana, tickberry, wild sage, is a low, erect, bushy, evergreen, wild shrub native to tropical and subtropical regions of the world with bunches of light purple flowers and opposite and whorled pubescent leaves 1. It is one of the world’s worst weed and a popular ornamental plant 2. It rapidly disturbed the ecological equilibrium due to its inexhaustible growth 3, 4.
The plant is used in folklore and traditional systems of medicine as a sudorific, intestinal antiseptic, diaphoretic and to treat asthma, abdominal disorders, bilious fever, cancer, catarrhal infections, chicken pox, eczema, hypertension, malaria, measles, swelling, rheumatism, tetanus and ulcers 5-8. It exhibited antimicrobial, insecticidal, nematicidal, immunosuppressive, antitumor 9, antimalarial 7, 10, antithrombin 11, anti-inflammatory, antinociceptive and antipyretic activities 12, 13. Previous phytochemical investigations of the plant showed the presence of steroids, flavonoids, camaryolic acid, methyl camera late and camangeloyl acid, steroid 14, fatty acids, triterpenoids 15-18, verbascoside 9, gautin, rutinoside, tricin, hispidulin, pectolinarigenin, icterogenin 19 and essential oils 20, 21.
This manuscript describes isolation and characterization of phytoconstituents from the leaves of L. indica grown in Delhi.
MATERIALS AND METHODS:
Materials: Melting points were determined on a thermoelectrically heated perfit apparatus without correction. The IR spectra were measured in KBr pellet on a Bio-Red FT-IR spectrometer. UV spectra were obtained in methanol with a Lambda Bio 20 spectrometer. The 1H (300 MHz) and 13C (75 MHz) NMR spectra were recorded on Bruker DRS 300 MHz spectrometer with TMS as an internal standard. Mass spectra were performed on a Jeol D-300 (EI/CI) system. Column chromatographic separations were carried out on silica gel (Merck, 60-120 mesh). Precoated silica gel plates (Merck, Silica gel 60 F254) were used for analytical thin layer chromatography, and the spots were visualized by exposure UV radiations and iodine vapors and spraying with ceric sulphate.
Plant Material: The fresh leaves of L. indica were collected from a field in New Delhi and identified by Prof. M.P. Sharma, Department of Botany, Jamia Hamdard. A specimen voucher of the leaves was deposited in the herbarium of the Phytochemistry Research Laboratory, Faculty of Pharmacy, Jamia Hamdard for future reference.
Preparation of Extract and Isolation: The dried pulverized leaves (1.5kg) were extracted with methanol in a Soxhlet extractor. The combined extracts were dried under reduced pressure to obtain a dark brown residue (145g). The residue (100g) was dissolved in minimum amount of methanol and adsorbed on silica gel for column grade (60-120 mesh) to prepare the slurry. It was air-dried, powdered and loaded on a silica gel column prepared in petroleum ether.
The column was run with petroleum ether (B.P. 60-80 °C), petroleum ether-chloroform (9:1, 3:1, 1:1, 1:3, v/v), chloroform and chloroform-methanol (99:1, 49:1, 19:5, 9:1, 17:3, 4:1, 7:3 and 1:1, v/v) mixtures. Various fractions were collected separately and matched by TLC to check homogeneity. Similar fractions having the same Rf values were combined and crystallized. The isolated compounds were recrystallized to get pure compounds as follows-
n-Hexadecanyl Oleate (1): Elution of the column with petroleum ether gave colourless crystals of 1, recrystallized from acetone - methanol, 1:1), 205 mg, m. p. 76-78 ºC, UV λmax (MeOH): 205 nm (log ε 4.5); IR νmax (KBr): 2928, 2859, 1731, 1645, 1458, 1372, 1215, 1078, 970, 765 cm-1, 1H NMR (CDCl3): δ 5.33 (1H, m, H-9), 5.01 (1H, m, H-10), 4.21 (2H, t, J = 6.1 Hz, H2-1ʹ), 2.79 (2H, t, J = 7.1 Hz, H2-2), 2.32 (2H, m, H2-8), 2.05 (2H, m, H2-11), 1.61 (2H, m, CH2), 1.52 (2H, m, CH2), 1.38 (4H, m, 2 x CH2), 1.32 (4H, m, 2 x CH2), 1.28 (10H, brs, 5 x CH2), 1.25 (28H, brs, 14 x CH2), 0.92 (3H, t, J = 6.7 Hz, Me-16), 0.87 (3H, t, J = 6.3 Hz, Me-18); ESI MS m/z (rel. int.): 506 [M]+ (C34H66O2) (4.7).
n-Octadecanyl Oleate (2): Elution of the column with petroleum ether - chloroform (3:1) yielded colourless crystals of 2, recrystallised from acetone - methanol, 1:1), 105 mg, m. p. 83 - 85 ºC; UV λmax (MeOH): 205 nm (log ε 3.8); IR νmax (KBr): 2927, 2859, 1720, 1635, 1441, 1375, 1219, 769 cm-1; 1H NMR (CDCl3): δ 5.35 (2H, m, H-9, H-10), 4.21 (2H, t, J = 6.6 Hz, H2-1ʹ), 2.80 (2H, t, J = 7.2 Hz, H2-2), 2.32 (2H, m, H2-8), 2.05 (2H, m, H2-11), 1.60 (2H, m, CH2), 1.56 (2H, m, CH2), 1.31 (16H, brs, 8 x CH2), 1.25 (340H, brs, 17 x CH2), 0.88 (3H, t, J = 6.6 Hz, Me-18′), 0.85 (3H, t, J = 6.5 Hz, Me-18); ESI MS m/z (rel. int.): 534 [M]+ (C36H70O2) (3.1).
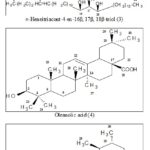
n-Heneitriacontenol (3): Elution of the column with chloroform produced a colourless amorphous powder of 3, recrystallized from acetone- methanol (1:1), 95 mg, m. p. 87 - 89 ºC, IR νmax (KBr): 3464, 3445, 2927, 2852, 1635, 1452, 1384, 990, 829, 726 cm-1; 1H NMR (CDCl3): δ 5.33 (2H, m, H-4, H-5), 3.89 (1H, m, w1/2 = 9.2 Hz, H-16α), 3.71 (1H, m, w1/2 = 8.5 Hz, H-17α), 3.67 (1H, m, w1/2 = 9.3 Hz, H-18α), 2.03 (2H, m, H2-3), 1.90 (2H, m, H2-6), 1.62 (2H, m, CH2), 1.55 (2H, m, CH2), 1.32 (4H, m, 2 x CH2), 1.28 (36H, brs, 18 x CH2), 0.89 (3H, t, J = 6.5 Hz, Me-1), 0.85 (3H, t, J = 6.3 Hz, Me-31); ESI MS (rel. int.): 482 [M]+ (C31H62O3) (38.1), 439 (5.2), 413 (37.9), 299 (8.1), 273 (9.8), 239 (10.2), 213 (9.5), 209 (6.2), 183 (7.8).
Oleanolic acid (4): Elution of the column with chloroform-methanol (49 : 1) afforded colourless crystals of 4, recrystallized from chloroform-methanol (1 : 1), 0.71 g, m. p. 307-309 ºC; UV λmax (MeOH): 209 nm (log ε 4.8); IR νmax (KBr): 3412, 2927, 2849, 1697, 1641, 1459, 1377, 1273, 1172, 1036, 995 cm-1; 1H NMR (CDCl3): δ 5.21 (1H, m, H-12), 3.18 (1H, dd, J = 5.6, 9.5 Hz, H-3β), 1.25 (3H, s, Me-23), 1.03 (3H, s, Me-25), 0.93 (3H, s, Me-24), 0.85 (3H, s, Me-30), 0.81 (3H, s, Me-29), 0.76 (3H, s, Me-27), 0.67 (3H, s, Me-26), 2.01- 1.17 (23 H, 10 x CH2, 3 x CH); 13C NMR (DMSO-d6): δ 38.55 (C-1), 28236 (C-2), 78.43 (C-3), 41.02 (C-4), 55.39 (C-5), 18.72 (C-6), 33.31 (C-7), 39.37 (C-8), 53.49 (C-9), 36.75 (C-10), 23.38 (C-11), 128.05 (C-12), 138.14 (C-13), 42.68 (C-14), 26. 75 (C-15), 28.56 (C-16), 41.55 (C-17), 50.49 (C-18), 45.71 (C-19), 31.28 (C-20), 34.61 (C-21), 31.16 (C-22), 28.79 (C-23), 15.17 (C-24), 15.68 (C-25), 27.32 (C-26), 21.84 (C-27), 178.36 (C-28), 24.72 (C-29), 26.19 (C-30); ESI-MS (rel.int.): 456 [M]+ (C30H48O3) (25.1), 438 (64.7), 411 (45.9), 393 (22.3), 248 (54.8), 207 (16.8), 205 (22.1), 203 (51.6), 189 (48.3), 174 (16.8).
Ursolic acid (5): Further elution of the column with chloroform-methanol (49:1) furnished colourless crystals of 5, recrystallized from chloroform - methanol (1 : 1), 0.55 g, m. p. 285-287 ºC; UV λmax (MeOH): 210 nm (log ε 4.8); IR νmax (KBr): 3427, 3231, 2934, 2845, 1695, 1645, 1451, 1375, 1224, 1189, 1091, 1033, 949, 815 cm-1; 1H NMR (CDCl3): δ 5.25 (1H, d, J = 7.1 Hz, H-12), 3.54 (1H, dd, J = 5.5, 9.3 Hz, H-3β), 1.25 (3H, s, Me-23), 1.13 (3H, s, Me-25), 0.98 (3H, s, Me-24), 0.89 (3H, d, J = 6.6 Hz, Me-30), 0.85 (3H, d, J = 6.6 Hz, Me-29), 0.79 (3H, s, Me-27 ), 0.67 (3H, s, Me-26), 2.03- 1.19 (23 H, 9 x CH2, 5 x CH); 13C NMR (CDCl3): δ 39.15 (C-1), 28.62 (C-2), 78.84 (C-3), 39.53 (C-4), 55.65 (C-5), 18.76 (C-6), 33.54 (C-7), 39.71 (C-8), 47.72 (C-9), 37.25 (C-10), 23.91 (C-11), 125.53 (C12), 139.71 (C-13), 42.42 (C-14), 28.39 (C-15), 24.63 (C-16), 47.49 (C-17), 53.12 (C-18), 42.09 (C-19), 40.11 (C-20), 32.26 (C-21), 35.27 (C-22), 29.15 (C-23), 15.73 (C-24), 15.89 (C-25), 16.89 (C-26), 23.67 (C-27), 179.12 (C-28), 18.16 (C-29), 22.53 (C-30); ESI-MS (rel.int.): 456 [M]+ (C30H48O3) (23.9), 438 (61.5), 411 (34.6), 393 (28.2), 248 (65.3), 207 (21.5), 203 (51.3), 189 (48.5), 174 (18.6).
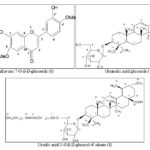
Lantanaflavone 7-O-β-D-glucoside (6): Elution of the column with chloroform-methanol (19:1) afforded pale yellow crystals of 6, recrystallized from methanol, 155 mg, m. p. 103-105 ºC; UV λmax (MeOH): 274, 331 nm; IR νmax (KBr): 3396, 3210, 2922, 2852, 1695, 1647, 1521, 1465, 1432, 1357, 1302, 1259, 1190, 1110, 1074, 1038, 915, 885 cm-1; 1H NMR (DMSO-d6): δ 8.07 (1H, d, J = 9.0 Hz, H-5′), 8.04 (1H, d, J = 9.0 Hz, H-6′), 7.15 (1H, s, H-5), 7.12 (1H, s, H-8), 7.04 (1H, s, H-2′), 6.96 (1H, s, H-3), 5.12 (1H, d, J = 7.2 Hz, H-1′′), 3.77 (1H, m, H-5′′), 3.69 (1H, m, H-2′′), 3.66 (1H, m, H-4′′), 3.50 (1H, m, H-3′′), 3.13 (2H, d, J = 6.8 Hz, H2-6′′), 3.74 (3H, brs, MeO - 6), 3.71 (3H, brs, MeO-4′); ESI MS m/z (rel.int.): 476 [M]+ (C23H24O11) (100), 328 (6.2), 300 (6.3), 176 (7.2), 179 (6.1), 163 (13.7), 137 (46.2), 123 (12.2), 120 (12.5).
Oleanolic acid 3-O-β-D-glucoside (7): Elution of the column with chloroform - methanol (9 : 1) afforded colourless crystals of 7, recrystallized from methanol, 265 mg, m .p. 216-217 ºC; UV λmax (MeOH): 213 nm (log ε 4.9); IR νmax (KBr): 3423, 3377, 3206, 2926, 2867, 1701, 1642, 1458, 1367, 1258, 1189, 1031, 875 cm-1; 1H NMR (DMSO-d6): δ 5.33 (1H, m, H-12), 5.09 (1H, d, J = 7.3 Hz, H-1′), 4.67 (1H, m, H-5′), 4.53 (1H, m, H-2′), 4.21 (1H, m, H-3′), 3.76 (1H, m, H-4′), 3.39 (1H, dd, J = 5.3, 9.5 Hz, H-3α), 3.04 (2H, d, J = 6.6 Hz, H2-6), 1.27 (3H, brs, Me-23), 1.05 (3H, brs, Me-25), 0.98 (3H, brs, Me-26), 0.95 (3H, s, Me-29), 0.93 (3H, brs, Me-30), 0.83 (3H, brs, Me-27), 2.27-1.39 (22 H, m, 9 × CH2, 4 x CH); 13C NMR (DMSO-d6): δ 39.25 (C-1), 28.21 (C-2), 77.81 (C-3), 39.83 (C-4), 55.39 (C-5), 18.35 (C-6), 32.29 (C-7), 39.94 (C-8), 50.81 (C-9), 37.18 (C-10), 23.32 (C-11), 122.51 (C-12), 139.34 (C-13), 39.78 (C-14), 26.61 (C-15), 28.61 (C-16), 42.85 (C-17), 50.39 (C-18), 45.11 (C-19), 30.08 (C-20), 31.11 (C-21), 29.98 (C-22), 22.75 (C-23), 15.17 (C-24), 16.81 (C-25), 27.39 (C-26), 23.12 (C-27), 179.16 (C-28), 26.77 (C-29), 27.49 (C-30), 102.56 (C-1′), 74.21 (C-2′), 67.51 (C-3′), 64.19 (C-4′), 79.78 (C-5´), 60.54 (C-6′). ESI MS m/z (rel.int.): 618 [M]+ (C36H58O8) (21.3), 455 (11.5), 438 (59.1), 411 (46.7), 393 (21.8), 378 (7.9), 248 (53.2), 207 (17.5), 205 (22.7), 203 (51.4), 189 (53.1), 174 (16.7).
Ursolic acid 3-O-β-D-glucosyl-6'-oleate (8): Further elution of the column with chloroform-methanol (9:1) afforded colourless crystals of 8, recrystallised from methanol, Rf m. p. 271-273 ºC; UV λmax (MeOH): 212 nm (log ε 5.1); IR νmax (KBr): 3424, 3361, 3245, 2926, 2849, 1725, 1707, 1636, 1451, 1382, 1278, 1239, 1181, 1035, 994, 729 cm-1; 1H NMR (CDCl3): δ 5.35 (1H, m, H-12), 5.32 (1H, m, H-9′′), 5.28 (1H, m, H-10′′), 5.09 (1H, d, J = 7.5 Hz, H-1′), 4.60 (1H, m, H-5′), 4.28 (1H, dd, J = 7.5, 6.0 Hz, H-2′), 4.15 (1H, m, H-3′), 3.96 (1H, m, H-4′), 3.95 (2H, d, J = 9.5 Hz, H2-6'), 3.63 (1H, dd, J = 5.5, 9.8 Hz, H-3α), 2.34 (2H, t, J = 7.5 Hz, H2-2′′), 2.16 (2H, m, H2-8''), 2.09 (2H, m, H2-11''), 1.65 (2H, m, CH2), 1.34 (6H, brs, 3 x CH2), 1.32 - 1.29 (9H, m, 5 x CH, 2 CH2), 1.25 (18H, brs, 9 x CH2), 1.22 (16H, brs, 8 x CH2), 1.09 (3H, brs, Me-23), 1.04 (3H, brs, Me-27), 1.01 (3H, brs, Me-25), 0.98 (3H, brs, Me-24), 0.87 (3H, brs, Me-26), 0.85 (3H, d, J = 6.6 Hz, Me-30), 0.82 (3H, t, J = 6.5 Hz, Me-18′′), 0.79 (3H, d, J = 6.6 Hz, Me-29); ESI MS (rel.int.): 882 [M]+ (C54H90O9) (3.8), 455 (21.2), 281 (35.2), 265 (8.3), 248 (11.2), 207 (18.5).
DISCUSSION: Compounds 1 and 2 were the fatty acid esters characterized as n- hexadecanyl oleate 22 and n-octadecanyl oleate 23, respectively. Compound 3 showed IR absorption bands for alcoholic groups (3464, 3445 cm-1), unsaturation (1635 cm-1) and long aliphatic chain (726 cm-1). Based on mass spectrum, its molecular mass was determined at m/z 482 corresponding to a molecular formula of a long chain aliphatic trihydroxy alcohol, C31H62O3. The ion peaks arising at m/z 439 [M – C3H7, C3 – C4 fission]+ and 413 [M-69, C5 – C6 fission]+ indicated the presence of the vinylic linkage at the C4 position. The ion fragments generated at m/z 209, 273 [C15 – C16 fission]+, 239 [C16 – C17 fission]+, 213 [C17 –C18 fission]+ and 183, 299 [C15 – C16 fission]+ suggested the existence of the hydroxyl groups at C16, C17 and C18 carbons. T
he 1H NMR spectrum of 3 showed a two - proton multiplet at δ 5.33 assigned to vinylic H-4 and H-5 protons. Three one-proton multiplets at δ 3.89 (w1/2 = 9.2 Hz, H-16α), 3.71 (w1/2 = 8.5 Hz, H-17α) and 3.67 (1H, m, w1/2 = 9.3 Hz, H-18α) were ascribed to α-oriented H-16, H-17 and H-18 carbinol protons, respectively. Four two-proton multiplets at δ 2.03, 1.90, 1.62 and 1.55, a four-proton multiplet at δ 1.32 and a broad singlet at δ 1.28 (28 H) were associated with the methylene protons. Two three-proton triplets at δ 0.89 (J = 6.5 Hz) and 0.85 (J = 6.3 Hz) were accounted to terminal primary C-1 and C-31 methyl protons, respectively. Based on the above discussion, the structure of 3 was characterized as n-heneitriacont-4-en-16β, 17β, 18β-triol. This is new aliphatic alcohol. Compounds 4 and 5 were the pentacyclic triterpenoids characterized respectively as oleanolic acid 24, 25 and ursolic acid 24, 26.
Compound 6, named lantanaflavone 7-O-β-D-glucoside, reacted positively to Shinoda and ferric chloride tests and showed UV absorption maxima at 274 and 331 nm distinctive for flavones 27, 28. There was no UV shift of band II on the addition of sodium acetate indicating bounded nature of the C-7 hydroxyl group. The absence of a bathochromic shift of band I on the addition of aluminum chloride ruled out the existence of free hydroxyl groups at C-5 and C-4′ position (Markham, 1982). The IR spectrum of 6 displayed characteristic absorption bands for hydroxyl groups (3396, 3210 cm−1), carbonyl function (1695 cm−1) and aromatic ring (1647, 1521, 1038 cm−1). Based on the mass spectrum, its molecular ion peak was established at m/z 476 consistent with a molecular formula of a flavone glycoside, C23H24O11.
The ion fragments arising at m/z 328 [C3,4 – C1,2 fission]+, 300, 176 [C4,10 – C1,9 fission]+ and 123 [C6H3(OH)(OMe)]+ indicated the presence of one each methoxy and glycoside units in ring A and one each of hydroxyl and methoxy groups in ring B. The ion peaks generated at m/z 179 [C6H11O6]+ and 163 [C6H11O5]+ suggested the location of a hexose moiety in the molecule. The 1H NMR spectrum of 6 displayed two one-proton doublets at δ 8.07 (J = 9.0 Hz) and 8.04 (J = 9.0 Hz) assigned to aromatic H-5′ and H-6′ protons, respectively, four one-proton singlets at δ 7.15, 7.12, 7.04 and 6.96 ascribed correspondingly to H-5, H-8, H-2′ and H-3 protons of the flavone, a one-proton doublet at δ 5.12 (J = 7.2 Hz) accounted to anomeric H-1′′ proton, other sugar protons from δ 3.77 to 3.13 and two three-proton singlets at δ 3.74 and 3.71 due to methoxy protons.
The 1H NMR values of 6 were compared with the related flavonoids 29, 30. Acid hydrolysis of 6 yielded D-glucose (Rf 0.12, n-butanol-acetic acid-water, 4:1:5) and a flavone unit. Based on the preceding discussion, the structure of 6 has been formulated as 6, 4'-dimethoxy-7, 3'-dihydroxyflavone-7-O-β-D-glucopyranoside, a new flavone glucoside.
Compound 7, [M]+ at m/z 618 (C36H58O8), showed IR absorption bands for hydroxyl groups (3415, 3385 cm-1), carboxylic function (3266, 1703 cm-1) and unsaturation (1645 cm-1). Its 1H NMR spectrum exhibited signals for a vinylic proton (δ 5.33, m, H-12), anomeric proton (δ 5.11, d, J = 7.3 Hz, H-1′), other sugar and H-3 oxymethine protons (δ 4.69 – 3.04) and seven tertiary methyl protons (δ 1.31- 0.83). The compound 7 was characterized as oleanolic acid 3-O-β-D-glucopyranoside 31, 32.
Compound 8 gave positive tests for triterpenic glycosides and showed characteristic IR absorption bands for hydroxyl groups (3424, 3361 cm-1), ester function (1725 cm-1), unsaturation (1636 cm-1) and long aliphatic chain (729 cm-1). Its molecular ion peak was determined based on mass and 13C NMR spectra at m/z 882 consistent to a molecular formula of a triterpenic glycosidic ester C54H90O9. The ion peaks arising at m/z 265 [C1′′ – O fission, CH3(CH2)7-CH=CH-(CH2)7 CO]+, 281 [C6′ – O fission, CH3(CH2)7-CH=CH- (CH2)7 COO]+ and 455 [O – C1′ fission, C30H47O3]+ indicated that oleic acid was linked to a hexose sugar unit which was attached to a pentacyclic triterpene. The ion peaks produced at m/z 207 and 248 due to Retro-Diels Alder fragmentation of the triterpenic unit suggested that a vinylic linkage was present at C12 carbon.
The 1H NMR spectrum of 8 exhibited three one- proton multiplets at δ 5.35, 5.32 and 5.28 assigned to vinylic H-12, H-9'' and H-10'' protons, respectively, a one – proton doublet at δ 5.09 (J = 7.5 Hz) ascribed to anomeric H-1' proton and other sugar protons as one-proton multiplets at δ 4.60 (H-5′), 4.15 (H-3′), 3.96 (H-4′), as a one – proton double doublet at δ 4.28 (J = 7.5, 6.0 Hz, H-2′) and as a two – proton doublet at δ 3.95 (J = 9.5 Hz) due to oxymethylene H2-6' protons. A one – proton double doublet at δ 3.63 (J = 5.5, 9.8 Hz) was accounted to oxymethine H-3α proton. Five three – proton singlets at δ 1.09, 1.04, 1.01, 0.98 and 0.87, two three – proton doublets at δ 0.85 (J = 6.6 Hz) and 0.79 (J = 6.6 Hz) and a three-proton triplet at δ 0.82 (J = 6.5 Hz) were associated with the tertiary C-23, C-27, C-25, C-24 and C-26, secondary C-30 and C-29 and tertiary C-18′′ methyl protons, all attached to the saturated carbons.
The other methine and methylene protons resonated from δ 2.34 to 1.25. The 1H NMR spectral data of the triterpenic unit of 8 were compared with spectral values of similar triterpenoids 33. Acid hydrolysis of 8 yielded ursolic acid (m. p. 285-287 ºC), D-glucose (Rf 0.12, n-butanol-acetic acid-water, 4:1:5) and oleic acid. On the basis of spectral data analysis and chemical reactions, the structure of 8 had been formulated as urs-12-en-3β-ol – 28 - oic acid 3β – D - glucopyranosyl -6´-octadecanoate. This is a new triterpenic glycosidic ester.
CONCLUSION: Phytochemical investigation of a methanolic extract of the leaves of L. indica resulted in the isolation of two fatty esters, one each of aliphatic alcohol and flavone glucoside and four pentacyclic triterpenoids. This work has enhanced understanding of the phytoconstituents of the plant. These compounds may be used as chromatographic markers for standardization of the plant leaves.
ACKNOWLEDGEMENT: The authors are thankful to the instrumentation centers, Central Drug Research Institute, Lucknow and Jawaharlal Nehru University, New Delhi for recording spectral data of the compounds.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Ganesh T, Saikatsen, Thilagam G, Loganatham T and Chakraborty R: Pharmacognostic and anti hyperglycaemic evaluation of Lantana camara (L.) var. Aculeate leaves in alloxan-induced hyperglycemic rats: Int J Res Pharm. 2010; 1(3): 247-252.
- Day MD, Willey CJ, Playford J and Zalucki MP: Lantana: current management status and future prospects; Australian Centre for International Agricultural Research: Canberra, Australian 2003.
- Juliania HRJ, Biurrun F, Koroch AR, Oliva MM, Demo MS, Trippi VS and Zygadlo JA: Chemical constituents and antimicrobial activity of the essential oil of Lantana xenica. Plant Medica 2002; 68: 759-762.
- Sharma OP, Makkar HP and Dawra RK: A review of the noxious plant Lantana camara. Toxicon 1988; 26: 975-987.
- Kirtikar KR and Basu BD: Indian Medicinal plants: Sri Satguru Indological and Oriental Publishers. Delhi 2000; 8: 2634-2636.
- Chopra RN, Nayar SL and Chopta IC: Glossary of Indian Medicinal Plants: CSIR, New Delhi 1999; 140-150.
- Ghisalberti EL: Lantana camara (Verbenaceae): Fitoterapia 2000; 71(5): 467-486.
- Abu-Shanab B, Adwan G, Jarrar N, Abu-Hijleh A and Adwan K: Antibacterial activity of four plant extracts used in Palestine in folkloric medicine against Methicillin-resistant Staphylococcus aureus. Turk J Biol 2006; 30: 195-98.
- Adiguzel A, Gulluce M, Sengul M, Ogutcu, Sahin F and Karaman I: Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turk J Biol 2005; 29: 155-60.
- Mahdi-Pour B and Sasidharan S: In-vivo toxicity study of Lantana camara. Asian Pac J Trop Biomed 2011; 1(3): 230-232.
- O’Neill MJ, Lewis JA, Noble HM, Holland S, Mansat C, Farthing JE, Foster G, Noble D, Lane SJ, Sidebottom PJ, Lynn SM, Hayes MV and Dix CJ: Isolation of translactone-containing triterpenes with thrombin inhibitory activities from the leaves of Lantana camara. J Nat Prod 1998; 61(11): 1328-1331.
- Uzcategui B, Avila D, Heberto SR, Quintero L, Ortega J and Gonzalez YB: Anti-inflammatory, antinociceptive and antipyretic effects of Lantana trifolia in experimental animals. Invest Clin 2004; 45(4): 317-322.
- Sagar L, Sehgal R and Ojha S: Evaluation of the antimotility effect of Lantana camara L. acuelata constituents on neostigmine induced gastrointestinal transit in mice. BMC Complement Altern Med 2005; 5: 18.
- Goyal MM and Kamal K: High content of cholesterol in the leaves of indica Roxb. Indian Drug 1984; 22: 41.
- Agarwal GP and Hasija SK: Microorganisms in the laboratory guide of Microbiology, Mycology and Plant Pathology: CBS publishers Lucknow (U.P) 1986; 1- 48.
- Singh SK, Tripathi VJ and Singh RH: Triterpenoids of Lantana indica (Verbenaceae). Indian Drugs 1984; 26: 395-400.
- Singh SK, Tripathi VJ and Singh RH: A new pentacyclic triterpene acid from Lantana indica. J Nat Prod 1998; 61: 1295.
- Ingawale GS and Giri ASG: Isolation and characterization of the bioactive molecule from Lantana camara. Asian J Research Chem 2014; 7(3): 339-344.
- Patil G, Khare AB, Huang KF and Lin FM: Bioactive chemical constituents from the leaves of Lantana camara Indian Journal of Chemistry 2015; 54B: 691-697.
- Singh G, Pandey SK, Leclerq PA and Sperkova J: Studies on essential oil, part 23: Chemical constituents of leaf oil of Lantana indica Roxburg from North India. J Essent Oil Res 2002; 4: 346- 347.
- Akhtar MS, Ali M, Madhurim and Mir SR: Chemical composition of essential oil of Lantana indica leaves. J Essent Oil Res 2006; 18: 611- 612.
- Najib S, Ahamad J, Ali M and Mir SR: Isolation and characterization of fatty acid esters from the seeds of Cichorium intybus. American Journal of Phytomedicine and Clinical Therapeutics 2014; 2(4): 469-473.
- Ali M, Naquvi KJ and Sultana S: Nonpolar chemical constituents from the Oryza sativa bran. Journal of Scientific and Innovative Research 2014; 3(6): 583-587.
- Hossain MA and Ismail Z: Isolation and characterization of triterpenes from the leaves of Orthosiphon stamineus. Arabian Journal of Chemistry 2013; 6: 295-298.
- Galgon T, Hoke D and Drager B: Identification and quantification of betulinic acid. Phytochem Anal 1999; 10: 187-194.
- Ali M and Sultana S: Phytochemical investigation of the leaves of Ficus pandurata Eurpean J Biomed Pharm Sci 2016; 3(10): 393-396.
- Harborne JB, Williams CA, Mabry TJ and Mabry H: The Flavonoids: Flavone and Flavonol Glycosides, Champmam and Hall London 1975; 376-441.
- Markham KR: Techniques of flavonoid identification, Academic Press; London, 1982; 36-46.
- Khalid SA and Waterman PG: 8C-prenylflavonoids from the seeds of Tephrosia bracteolate. Phytochemistry 1981; 20(7): 1719-1720.
- Yadav DK, Ali M, Ghosh AK and Kumar B: Isolation of flavonoid from Abies webbiana leaves and its activity. Pharmacognosy J 2016; 8(4): 341-345.
- Ghosh D, Thejomoorthy P and Veluchamy: Anti-inflammatory and analgesic activities of oleanolic acid 3-/3- glucoside (RDG-1) from Randia dumetorum (Rubiaceae). Indian J Pharmacology 1983; 15(4): 331-342.
- Ali M, Sultana S and Mir SR: Aliphatic alcohols, alkylated aromatic and triterpenic constituents from the aerial parts of Rhus chinensis J Pharm Biol Sci 2017; 5(1): 1-7.
- Ali M: Techniques in terpenic identification, Birla publications Delhi 2001.
How to cite this article:
Ali M, Jameel M, Mir SR and Sultana S: Chemical constituents from the aerial parts of Lantana indica roxb. Int J Pharmacognosy 2017; 4(6): 193-99. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.4(6).193-99.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
193-199
603
1320
English
IJP
M. Ali *, M. Jameel, S. R. Mir and S. Sultana
Phytochemistry Research Laboratory, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India.
maliphyto@gmail.com
08 March 2017
21 May 2017
30 May 2017
10.13040/IJPSR.0975-8232.IJP.4(6).193-99
01 June 2017