CHEMICAL CONSTITUENTS AND BIOLOGICAL ACTIVITIES OF GENUS RUELLIA
HTML Full TextCHEMICAL CONSTITUENTS AND BIOLOGICAL ACTIVITIES OF GENUS RUELLIA
Mamdouh Nabil Samy 1, * 2, Sachiko Sugimoto 2, Katsuyoshi Matsunami 2, Hideaki Otsuka 2, 3 and Mohamed Salah Kamel 1
Department of Pharmacognosy 1, Faculty of Pharmacy, Minia University, Minia 61519, Egypt.
Department of Pharmacognosy 2, Graduate School of Biomedical Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8553, Japan.
Department of Natural Products Chemistry 3, Faculty of Pharmacy, Yasuda Women's University, 6-13-1 Yasuhigashi, Asaminami-ku, Hiroshima 731-0153, Japan.
ABSTRACT: The genus Ruellia L. is sometimes called Dipteracanthus, it comprises about 150 species native to tropical and temperate North and South America. In this review, the literature data on phytochemical and biological investigations of the genus Ruellia are compiled. The well-recognized groups of secondary metabolites were flavonoids, lignans, coumarins, alkaloids, triterpenes, sterols, phenolic glycosides, phenylethanoids, megastigmane glycosides, benzoxazinoid glucosides, and others. The extract of this genus as well as pure compounds isolated from it have been demonstrated to possess multiple pharmacological activities such as wound healing, cardio-vascular, anti-hyperglycemic, antioxidant, antimicrobial, antibacterial, anticancer, antinociceptive, anti-inflammatory, cytotoxic and gastro-protective activities, purgative and angiotensin-converting enzyme-inhibitory effects, estrogenic and cholinergic properties and anti-fertility action.
| Keywords: |
Ruellia, Acanthaceae, Chemical constituents, Biological activities
INTRODUCTION: The family Acanthaceae (Acanthus family) is a large plant family, includes about 250 genera with almost 2500 species mostly found in hot countries, tropical and subtropical regions of the world, and also found in Mediterranean regions, Australia and USA 1-4. Some species of the family Acanthaceae are used in folk medicine to treat several diseases, especially gastrointestinal ailments 5.
Some plants are used as purgative, emetic, in childbirth to relieve pain, food stuff, and diuretic, 2, 6, 7 antidysentric, galactagogue and antidote for snake-bite, while being used externally as a poultice in rheumatism 2, 6. The genus Ruellia L. is sometimes called Dipteracanthus; it comprises about 150 species native to tropical and temperate North and South America. Some species of genus Ruellia are used medicinally to treat gonorrhea, syphilis, eye sores and in renal infections 7-9. Economically, many members of the family Acanthaceae are used in blue and yellow dye manufacture 10.
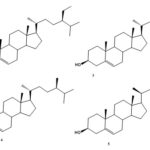
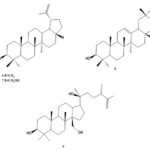
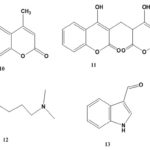
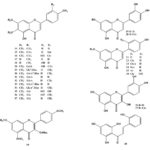
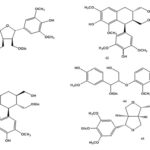
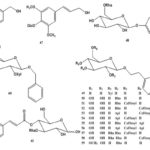
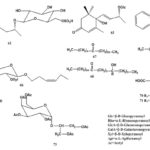
Chemical Constituents: The chemical constituents of genus Ruellia include flavonoids, lignans, coumarins, alkaloids, triterpenes, sterols, phenolic glycosides, phenyl ethanoids, megastigmane glycosides, β-Sitosterolglucoside, and others. Their structures, 1 - 70 are shown below, and their names and the corresponding plant sources are collected in Table 1 and Fig. 1. As can been seen, flavonoid glycosides are the predominant constituents within the genus Ruellia.
Sterols: Five phytosterols, β-Sitosterol (1), β-sitosterol glucoside (2), stigmasterol (3), campesterol (4) and stimat-6-en-3-β-ol(5) have been isolated from the genus Ruellia 11-18.
Triterpenes: Four triterpenes (6-9) were isolated from the genus Ruellia 13-15, 19, 20. Most of the triterpenoids are pentacyclic, in addition to one tetracyclic triterpenoid was found in from R. tuberose 20.
Coumarins: Only two coumarins, (10, 11), were isolated from R. patula 18.
Alkaloids: Two alkaloids, (12, 13), were obtained from the plants of the genus Ruellia 19, 21.
Flavonoids: Flavonoids are the predominant secondary metabolites of Ruellia. 27 compounds, 14-37, were obtained from the genus Ruellia 14-17, 19, 22-24. Apigenin, luteolin, pectolaringenin, demethoxycentaureidin, and nepetin and their glycosides are the most common flavones isolated from plants of the genus Ruellia. Chalcone and flavonols quercetin and quercetin 3-O-glucoside were obtained from R. brittoniana 14.
Lignans: Four lignans (41-44) and one neolig-nan (45) were isolated from genus Ruellia 15-17, 26.
Phenolic glycosides: Four phenolic glycosides (46-49) were obtained from the genus Ruellia 16, 17.
Phenyl Ethanoids: Structurally, they are characterized by (hydroxyphenyl) ethyl moieties, and a p-caffeoyl groups attached to C-1’, and C-4’ and C-6’ of the glucose moiety through glycoside and ester linkages, respectively. Rhamnose may also be attached to the glucose residue. Twelve phenylethanoids, 50-61, were found in the genus Ruellia 16, 17, 24.
Megastigmanes: Only two megastigmane glucosides, byzantionoside B 6'-O-sulfate (62) and (6S,9R)-rose side (63), were isolated from R. Patula and R. tuberose 16, 17.
Benzoxazinoids: Two benzoxazinoids (64, 65) were found in R. tuberose 17.
Other Constituents: Aliphatic compounds and aliphatic alcohol glycosides (66-73) were obtained from the genus Ruellia 14, 16, 19, 27, 28.
TABLE 1: CHEMICAL CONSTITUENTS FROM THE GENUS RUELLIA
| S. no. | Class and Name | Source | Ref. |
| Sterols | |||
| 1 | β-Sitosterol
|
R. tuberosa
R. prostrata R. tuberosa R. brittoniana |
11
12 13 14 |
| 2 | β-Sitosterolglucoside | R. patula
R. tuberosa R. brittoniana |
15, 16
17 14 |
| 3 | Stigmasterol
|
R. tuberosa
R. prostrata R. tuberosa R. patula |
11
12 13 18 |
| 4 | Campesterol
|
R. tuberosa
R. tuberosa R. patula |
11
13 18 |
| 5 | Stimat-6-en-3-β-ol | R. patula | 18 |
| Triterpenes | |||
| 6 | Lupeol | R. tuberosa
R. brittoniana R. patula |
13
14 15 |
| 7 | Betulin | R. tuberosa | 19 |
| 8 | β-Amyrin | R. brittoniana | 14 |
| 9 | 21-Methyldammar-22-en-3β,18,27-triol | R. tuberosa | 20 |
| Coumarins | |||
| 10 | 7-Hydroxy-4-Methyl Coumarin | R. patula | 18 |
| 11 | Dicoumarol | R. patula | 18 |
| Alkaloids | |||
| 12 | Tetramethylputrescine | R. rosea | 21 |
| 13 | Indole-3-carboxaldehyde | R. tuberosa | 19 |
| Flavonoids | |||
| 14 | Cirsimaritin | R. tuberosa | 19 |
| 15 | Cirsimarin | R. tuberosa | 19 |
| 16 | Cirsiliol 4′-glucoside | R. tuberosa | 19 |
| 17 | Sorbifolin | R. tuberosa | 19 |
| 18 | Pedalitin | R. tuberosa | 19 |
| 19 | Luteolin | R. prostrate | 22 |
| 20 | Luteolin 7-O-glucoside | R. prostrata
R. tuberosa |
22
23 |
| 21 | Apigenin | R. prostrate
R. brittoniana |
22
14 |
| 22 | Apigenin 7-O-glucoside | R. prostrata
R. tuberosa R. brittoniana |
22
23 14 |
| 23 | Apigenin 7-O-glucuronide | R. prostrata
R. tuberosa |
22
23 |
| 24
25 |
Apigenin 7-O-rutinoside | R. tuberosa
R. patula |
23
15 |
| 7-O-Acetyl apigenin | R. brittoniana | 14 | |
| 26 | Quercetin | R. brittoniana | 14 |
| 27 | Quercetin 3-O-glucoside | R. brittoniana | 14 |
| 28 | Demethoxycentaureidin 7-O-β-D-galacturonopyranoside | R. patula | 16 |
| 29 | Pectolinarigenin 7-O-α-L-rhamnopyranosyl-(1'''→4'')-β-D-glucopyranoside | R. patula | 16 |
| 30 | Pectolinarigenin 7-O-α-L-rhamnopyranosyl-(1'''→4'')-β-D-glucuronopyranoside | R. patula | 16 |
| 31 | Hispidulin 7-O-β-D-glucuronopyranoside | R. tuberosa | 24 |
| 32 | Comanthoside B | R. tuberosa | 24 |
| 33 | Hispidulin
7-O-α-L-rhamnopyranosyl-(1'''→2'')-O- β -D-glucuronopyranoside |
R. tuberosa | 24 |
| 34 | Pectolinaringenin 7-O- α -L-rhamnopyranosyl-(
1'''→2'')-O- β -D-glucuronopyranoside |
R. tuberosa | 24 |
| 35 | Nepetin7-O-β-D-glucopyranoside | R. tuberosa | 17 |
| 36 | Demethoxycentaureidin 7-O-β-D-glucopyranoside | R. tuberosa | 17 |
| 37 | Pectolinarigenin 7-O- β-D-glucopyranoside | R. tuberosa | 17 |
| 38 | 5, 2′, 3′ -trihydroxy 7-O-glucoflavone | R. brittoniana | 25 |
| 39 | 5, 7, 4′ -trimethoxy 3-O-Rhamnopyranoside | R. brittoniana | 25 |
| 40 | 2, 2′, 4′, 6′-tetrahydroxy-chalcone | R. brittoniana | 25 |
| Lignans | |||
| 41 | 5,5′-Dimethoxylariciresinol 9-O-β-D-glucopyranoside (Rupaside) | R. patula | 26 |
| 42 | (+)-Lyoniresinol-9′-O-β-D-glucopyranoside | R. patula
R. brittoniana |
26, 16
15 |
| 43 | (−)-Lyoniresinol 3α-O-β-D-glucopyranoside | R. tuberosa | 17 |
| 44 | 3-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxy-1-(E)-propenyl)-2-methoxyphenoxy] propyl-β-D-glucopyranoside | R. tuberosa | 17 |
| 45 | syringaresinol 4,4'-O-bis-β-D-glucopyranoside. | R. tuberosa | 17 |
| Phenolic compounds | |||
| 46 | Vanilloside | R. patula | 16 |
| 47 | Syringin | R. patula
R. tuberosa |
16
17 |
| 48 | 3,4,5-Trimethoxyphenol O-α-L-rhamnopyranosyl-(1''→6')-β-D-glucopyranoside | R. patula | 16 |
| 49 | Benzyl alcoholO-β-D-xylopyranosyl-(1''→2')-β-D-glucopyranoside | R. patula | 16 |
| Phenyl ethanoids | |||
| 50 | Phenethyl alcohol-β-D-xylopyranosyl (1''→2')-β-D-glucopyranoside | R. patula | 16 |
| 51 | Bioside(decaffeoylverbascoside) | R. patula | 16 |
| 52 | Acteoside | R. patula
R. tuberosa |
16
17, 24 |
| 53 | Isoacteoside | R. patula
R. tuberosa |
16
24 |
| 54 | Nuomioside | R. tuberosa | 24 |
| 55 | Isonuomioside | R. tuberosa | 24 |
| 56 | Forsythoside B | R. tuberosa | 24 |
| 57 | Paucifloside | R. tuberosa | 24 |
| 58 | Cassifolioside | R. tuberosa | 24 |
| 59 | Isocassifolioside | R. tuberosa | 24 |
| 60 | Cistanoside E | R. patula | 16 |
| 61 | Cistanoside F | R. tuberosa | 17 |
| Megastigmanes | |||
| 62 | Byzantionoside B 6'-O-sulfate | R. patula | 16 |
| 63 | (6S,9R)-Roseoside | R. patula
R. tuberosa |
16
17 |
| Benzoxazinoids | |||
| 64 | HBOA-Glc | R. tuberosa | 17 |
| 65 | DIBOA-Glc | R. tuberosa | 17 |
| Others | |||
| 66 | (Z)-Hex-3-en-1-ol O-β-D-xylopyranosyl-(1''→2')-β-D-glucopyranoside | R. patula | 16 |
| 67 | Tritriacontan-6-one | R. tuberosa | 27 |
| 68 | 5-Hydroxytetratriacontan-9-one | R. tuberosa | 27 |
| 69 | n-Tritriacontane | R. tuberosa | 27 |
| 70 | Vanillic acid | R. tuberosa | 19 |
| 71 | p-Methoxy benzoic acid | R. brittoniana | 14 |
| 72 | (Z)-p-Coumaric acid | R. brittoniana | 14 |
| 73 | 2-O-α-Galactopyranoyl glycerol hexaacetate | R. brittoniana | 28 |
FIG. 1: STRUCTURES OF THE ISOLATED COMPOUNDS FROM GENUS REULLIA
Biological Activities: Reviewing the available literature about the genus Ruellia showed that it had the following biological activities:
Wound Healing Activity: The methanolic extract of Dipteracanthuspatulus promoted wound healing activity in albino rats by increasing cellular proliferation and formation of granulation tissue 29.
Cardiovascular Activity: The crude extract and aqueous and 1-butanolic fractions of R. patula and R. brittoniana displayed hypertensive effect and possessed cardiotonic properties on isolated rabbit's heart 30.
Anti-hyperglycemic Activity: The hypoglycemic activity of R. Tuberose was determined by oral administration of methanol extract and n-hexane and ethyl acetate fractions to normal and diabetic rabbits. Diabetes was induced by intraperitoneal injection of alloxan monohydrate (150 mg/kg body wt.). Optimum dose (500 mg/kg) of R. tuberosa to normal and diabetic rabbits showed significant blood glucose lowering effect. Ethyl acetate fraction (100 mg/kg) showed the highest anti-diabetic activity with 34.31 ± 0.43% (P<0.005) decrease in glycemia, while n-hexane fraction (150 mg/kg) showed moderate anti-diabetic activity and lowered the blood glucose level around 15.17 ± 0.58 % (P<0.005). The results were compared with the std. drug tolbutamide (100 mg/kg) 31.
50% Hydroethanolic leaf extracts of R. tuberosa and Diptera canthuspatulus at 500 mg/kg body weight possessed anti-hyperglycemic activity in Wistar albino rats 32, 33.
Antinociceptive and Anti-Inflammatory Properties: The ethanolic extract of R. tuberosa had antinociceptive and anti-inflammatory properties in experimental mice and rat of a dose of 300 mg/kg in the hot-plate test 34.
Antioxidant Activity: 50% Hydroethanolic leaf extracts of R. tuberosa and Dipteracanthuspatulus at 500 mg/kg body weight possessed antioxidant activity 32, 33. Ethyl acetate and chloroform fractions of the stem of R. tuberosa possessed potent antioxidant activity compared with methanolic extract and aqueous and n-hexane fractions, which was investigated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and the hydrogen peroxide-induced luminal chemiluminescence assay 35. Compound 36 showed noticeable DPPH radical scavenging activities (with IC50 value of 14.3 ± 1.10 µM), while compounds 40, 41, 46 and 50 exhibited a moderate activity (with an IC50 value of 37.5 ± 2.20, 31.9 ± 3.35, 31.7 ± 2.47 and 19.4 ± 2.59 µM, respectively). On the other hand, EtOAC fraction of R. patula displayed activity with IC50 25.5 ± 2.29 µg/ml compared with the standard trolox 16.7 ± 1.86. 36
Cytotoxicity: Compounds 14 and 15, which were isolated from R. tuberosa showed cytotoxicity in-vitro against KB cell line with the dose of 30.05 and 17.91 μg/ml, respectively, while cirsimarin was cytotoxic against HepG2 cell line with an IC50 value of 38.83 μg/ml.19
Methanolic extract of aerial part of R. tuberose possessed cytotoxicity. The minimum inhibitory concentration (IC50) for methanolic extract was found to be 3.5 and 1.9 μg/ml in H460 and MDA-MB231 cancer cells, respectively 37. Methanolic extract, n-hexane and EtOAc fractions of R patula, and MeOH extract, n-hexane and EtO Acfractions of R. tuberose exhibited significant cytotoxic activity at a concentration of 100μM (μg/ml) against human lung cancer cell lines A459, as compared with the positive control, doxorubicin 36.
Antibacterial Properties: The chloroform, ethyl acetate, alcohol and aqueous extracts of the whole plant of R. tuberosa showed significant antibacterial properties. The aqueous extract exhibited less activity against fungal organisms 38.
The methanol leaf extract of R. tuberosa showed significant antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Bacillus subtilis, Proteus mirablis and antifungal activity against Aspergillus sp., Mucor sp., Penicillium sp. and Fusarium sp. The antibacterial potential of R. tuberosa methanol extract was tested by using Agar well diffusion method. The (100 mg /mL) leaf extract showed maximum inhibition against Proteus mirablis (7 mm) . Further, the extract showed the maximum zone of inhibition against the fungus of Aspergillus sp. (8mm) 39 .
Gastroprotective Activity: Aqueous extract of R. tuberose roots showed a dose-dependent and robust gastroprotective activity in an alcohol-induced gastric lesion model of rats. The extract also had mild erythropoietic and moderate analgesic activities and was well tolerated even with subchronic treatment 40.
Purgative Effect: The methanol, ethyl acetate and aqueous extracts of R. praetermissa initiated spontaneous contractions in the quiescent and increased contraction on the electrically stimulated ileal strip at a concentration of 30 µg/ml. The extracts produced concentration-related contractions both in amplitude and tone up till 750 µg/ml with IC50 of 360 µg/ml (methanol extract), 425 µg/ml (ethyl acetate extract) and 540 µg/ml (aqueous extract) 41.
Angiotensin-Converting Enzyme-Inhibitory Effect: n-Hexane, ethyl acetate, methanol and aqueous extracts of R. praetermissa showed various inhibitory effects on ACE at a concentration of 0.33 mg/ml 42.
Estrogenic and Cholinergic Properties: R. praetermissa possessed direct influence on the uterine physiology during gestation in rats. The plant extract appears to activate the myometrial cells membrane muscarinic receptors resulting in a uterotonic effect by a mode of action possibly via the cholinergic system. The extract is possibly acting by facilitating the synthesis of endogenous estradiol which influences the stimulation of the growth of the uterine endometrium 43.
Antifertility Action: The aqueous extract of R. prostrata had a 40% antifertility action in female rats at a dose of 500 mg/kg, and the aqueous and petroleum ether extracts at a dose of 100 mg/kg had a 20% antifertility action. The ethanolic extract had no activity 44.
Anti-leishmanial Activity: It was found that n-hexane fraction of R. tuberosa showed weak inhibitory activity at 100 µg/ml, as compared with the positive control, amphotericin B 36.
CONCLUSION: The genus Ruellia is widespread all over the world, and many species of this genus have been used in traditional folk medicine. Phytochemical investigations of Ruellia species have revealed that many components from this genus exhibit significant biological and pharmacological activities. The typical constituents of this genus are flavonoids, lignans, and phenylethanoids. Although there are 250 species in this genus, only a few species have been investigated so far. Further phytochemical and biological studies should be carried out on this genus to elucidate their active principles and mechanisms of action of the active constituents.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Chopra GL: Angiosperms. Jullundur: S. Sagin 1973.
- Bailey LH: The Standard Cyclopedia of Horticulture, the MacMillan Company, Vol. I. 1963.
- Willis JC: A Dictionary of the Flowering Plants and Ferns. Cambridge University Press: London, New York, New Rochelle, Melbourne Sydney, Eighth Edition 1973.
- Trease GE, Evans: Pharmacognosy. Baillere and Tindall Press: London, Fifteenth Edition 2002.
- Luciane FB, Felipe TG, Sidnei BOF and Fabio SM: Dirhamnosyl flavonoid and other constituents from Brillantaisia palisatti. Quimica Nova 2003; 26:922-923.
- Watt JM, Breyer-Brandwiik MG: The Medicinal and Poisonous Plants of Southern and Eastern Africa. E.&S. Livingstone LTD: Edinburgh and London, Second Edition 1962.
- Kirtikar KR and Basu BD: Indian Medicinal Plants Jayyed Press: Delhi, Vol. III. Second Edition 1975.
- Nadkarni AK and Chopra RN: Indian Materia Medica, Popular Book Depot: Bombay 1954.
- Harborne JB: Phytochemical Methods. Chapman and Hall LTD: London, New York, Second Edition 1973.
- Metcalfe CR and Chalk L: Anatomy of the Dicotyledons. Clarendon Press: Oxford, Vol. II 1972.
- Behari M, Goyal MM and Streibl M: Natural products from Ruellia tuberosa L. Journal of Indian Chemical Society 1981; 58: 176-177.
- Banerjee AK: Sterols from Ruellia prostrata Current Science 1984; 53:144-145.
- Andhiwal CK, Has C and Varshney RP: Hydrocarbons, lupeol and phytosterols from the tubers of Ruellia tuberosa Indian Drugs 1985; 23: 48-49.
- Gobraeil LG: Pharmacognostical study of Ruellia brittoniana Leonard family Acanthaceae cultivated in Egypt. Master Thesis, Department of Pharmacognosy, Faculty of pharmacy, Assuit University 2009.
- Akhtar MF: Chemical and biological investigations of medicinal herbs, Phyla nodiflora, Ruellia patula and Ruellia brittoniana. Ph. D. thesis, Department of Pharmacognosy, Faculty of Pharmacy, Karachi University, Pakistan 1993.
- Samy MN, Khalil HE, Sugimoto S, Matsunami K, Otsuka H and Kamel MS: Three new flavonoid glycosides, byzantionoside B 6'-O-sulfate and xyloglucoside of (Z)-hex-3-en-1-ol from Ruellia patula. Chemical & Pharmaceutical Bulletin 2011; 59: 725-729.
- Samy MN, Khalil HE, Wanas AS, Kamel MS, Sugimoto S, Matsunami K and Otsuka H: Chemical constituents from the leaves of Ruellia tuberosa. Chemistry of Natural Compounds 2013; 49: 175-176.
- Muthumani P, Venkatraman S, Meera R, Devi P, Kameswari B and Eswarapriya B: Phytochemical investigation of Ruelia patula, Luffa cylindrica and Llephantopus scaber. Der Pharma Chemical 2009; 1: 210-218.
- Lin C, Huang Y, Cheng L, Sheu S and Chen C: Bioactive flavonoids from Ruellia tuberosa. Journal of Chinese Medicine 2006; 17: 103-109.
- Singh RS, Pandey HS, Pandey RP and Singh BK: A new triterpenoid from Ruellia tuberosa Journal of Chemistry 2002; 41B: 1754-1756.
- Johne S, Gröger D and Radeglia R: Tetramethylputrescine from young plants of Ruellia rosea. Phytochemistry 1975; 14: 2635-2636.
- Subramanian SS and Nair AGR: Flavonoids of Ruellia prostrata and Barleria cristata. Journal of Indian Chemical Society 1972; 49: 825-826.
- Nair AGR and Subramanian SS: Apigenin glycosides from Thunbergiafragrans and Ruellia tuberosa. Current Science 1974; 43: 480.
- Phakeovilay C, Disadee W, Sahakitpichan P, Sitthimonchai S, Kittakoop P, Ruchirawat S and Kanchanapoom T: Phenylethanoid and flavone glycosides from Ruellia tuberosa Journal of Natural Medicine 2003; 67:228-233.
- Elgindi MR, Hagag EG and Mohamed SE: Phytochemical and Biological Studies of Ruellia brittoniana. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2015; 6: 926-933.
- Ahmad M, Akhtar MF, Miyase T, Ueno A, Rashid S and Usmanghani K: Studies on the medicinal herb Ruellia patula. Pharmaceutical Biology 1993; 31: 121-129.
- Misra TN, Singh RS, Pandey HS, Pandey RP and Singh BK: Two new aliphatic compounds from Ruellia tuberosa Indian Journal of Chemistry 1997; 36B: 1194-1197.
- Ahmad VU, Choudhary MI, Akhtar MF, Ahmed M, Rizwani GH, Usmanghani K and Clardy J: 2-O-α-D-galactopyranosyl glycerol hexaacetate from Ruellia brittoniana. Journal of Natural Products 1990; 53: 960-963.
- Saroja K, Elizabeth JD and Gopalakrishnan S: Wound healing activity of the leaves of Dipteracanthuspatulus (Jacq.) Nees. Pharmacologyonline, 2009; 2: 462-469.
- Akhtar MF, Rashid S, Ahmad M and Usmanghani K: Cardiovascular evaluation of Ruellia patula and Ruellia brittoniana. Journal of Islamic Academy Sciences 1992; 5: 67-71.
- Ullah S, Shahwar D, Ullah S, Ahmad M and Ahmad N: Hypoglycemic activity of Ruellia tuberosa Linn (Acanthaceae) in normal and alloxan-induced diabetic rabbits. Journal of Chemical Society Pakistan 2012; 34: 436-441.
- Manikandan A and Victor ADD: Antimicrobial and antioxidant properties of 50% hydroethanolic leaf extracts of Ruellia tuberosa and Dipteracanthus patulus (Jacq.) leaves. Journal of Pharmacology 2009; 1: 45-49.
- Manikandan A and Victor ADD: Effect of 50% hydroethanolic leaf extracts of Ruellia tuberosa and Dipteracanthus patulus (Jacq.) on non-enzymic antioxidants and other biochemical parameters in the liver, kidney, serum of alloxan-induced diabetic swiss albino rats. Journal of Biomedical Science & Research 2010; 2: 182-193.
- Alam MA, Subhan N, Awal MA, Alam MS, Sarder M, Nahar L and Sarker SD: Antinociceptive and anti-inflammatory properties of Ruellia tuberosa. Pharmaceutical Biology 2009; 47: 209-214.
- Chen FA, Wu AB, Shieh P, Kuo DH and Hsieh CY: Evaluation of the antioxidant activity of Ruellia tuberosa. Food Chemistry 2006; 94: 14-18.
- Samy MN, Khalil HE, Sugimoto S, Matsunami K, Otsuka H and Kamel MS: Biological studies on chemical constituents of Ruellia patula and Ruellia tuberosa. Journal of Pharmacognosy and Phytochemistry 2015; 4: 64-67.
- Chothani DL, Patel MB, Mishra SH and Vaghasiya HU: Review on Ruellia tuberosa (Cracker plant). Pharmacognosy Journal 2010; 2: 506-512.
- Arirudran B, Saraswathy A and Krishnamurthy V: Antimicrobial Activity of Ruellia tuberosa (Whole Plant). Pharmacognosy. Journal 2001; 3: 91-95
- Senthilkumar P, Sambath R and Vasantharaj S: Antimicrobial potential and screening of antimicrobial compounds of Ruellia tuberose using GC-MS. International Journal of Pharmaceutical Sciences Review and Research 2013; 20: 184-188.
- Arambewela LSR, Thambugala R and Ratnasooriya WD: Gastroprotective activity of Ruellia tuberosa root extract in rats. Journal of tropical medicinal plants 2003; 4: 191–194.
- Salah AM, Dongmo AB, Kamanyi A, Bopelet M, Vierling W and Wagner H: In-vitro purgative effect of Ruellia praetermissa. Sceinf. ex. Lindau (Acanthaceae). Journal of Ethnopharmacology 2000; 72: 269-272.
- Salah AM, Dongmo AB, Kamanyi A, Bopelet M and Wagner H: Angiotensin-converting enzyme-inhibitory effect by Ruellia praetermissa. Pharmaceutical Biology 2001; 39: 16-19.
- Salah AM, Gathumbi J, Vierling W and Wagner H: Estrogenic and cholinergic properties of the methanol extract of Ruellia praetermissa ex. Lindau (Acanthaceae) in female rats. Phytomedicine 2002; 9: 52-55.
- Andhiwal CK, Has C and Varshney RP: Antifertility screening and phytochemical investigation of Ruellia prostrata Journal of Indian Chemical Society 1986; 63: 934.
How to cite this article:
Samy MN, Sugimoto S, Matsunami K, Otsuka H and Kamel MS: Chemical constituents and biological activities of genus Ruellia. Int J Pharmacognosy 2015; 2(6): 270-79. doi: 10.13040/IJPSR.0975-8232.2(6).270-79.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
270-279
828
3913
English
IJP
M. N. Samy *, S. Sugimoto, K. Matsunami, H. Otsuka and M. S. Kamel
Department of Pharmacognosy, Graduate School of Biomedical Sciences, Hiroshima University, Kasumi, Minami-ku, Hiroshima, Japan.
mamdouhnabil.2006@yahoo.com
30 April 2015
24 June 2015
28 June 2015
10.13040/IJPSR.0975-8232.IJP.2(6).270-79
30 June 2015