CHEMICAL COMPOSITION OF FIXED OIL FROM THE SEEDS OF SANTALUM RUBRUM BY GC-MS
HTML Full TextCHEMICAL COMPOSITION OF FIXED OIL FROM THE SEEDS OF SANTALUM RUBRUM BY GC-MS
Shahin Aziz * 1, Tahmina Khondkar Mitu 2 and Sharif M. Al-Reza 2
Chemical Research Division 1, BCSIR Laboratories, Dhaka - 1000, Bangladesh.
Department of Applied Chemistry and Chemical Engineering 2, Islamic University, Kushtia - 7003, Bangladesh.
ABSTRACT: Santalum rubrum is medicinally important plant belonging to the family of Santalaceae. It has been used extensively by Ayurvedic practitioner and is also reported to contain anthraquinones and flavonoids, these constituents are well documented to possess anti-inflammatory activity. The fatty acid compositions of the petroleum ether extract of seeds of this plant were determined by Gas Chromatography-Mass Spectrophotometer. 7 compounds were identified from the extract of seeds (99.97 %), and they are palmitic acid, stearic acid, oleic acid, linoleic acid, arachidonic acid, behenic acid and lignoceric acid of methyl ester. Among all fatty acids of linoleic acid showed the highest concentration (38.44%).
| Keywords: |
Santalum rubrum, GC-MS, Fatty acid compositions
INTRODUCTION: Medicinal plant products have been part of phytomedicine since time immemorial. These can be derived from any part of the plant like leaves, flowers, bark roots, fruits, and seeds, etc. 1 Any part of the plant may contain active components. Herbal medicines have become more popular in the treatment of any diseases due to popular belief that green medicine is safe, easily available and with fewer side effects. Many plants are cheaper and more accessible to most people especially in the developing countries than orthodox medicine, and there is a lower incidence of adverse effects after use. These reasons might account for their worldwide attention and use 2. The medicinal properties of some plants have been documented by some researchers 3, 4, 5.
Medicinal plant constitutes the main source of new pharmaceuticals and healthcare product 6. Extraction and characterization of several phyto-compounds of these green factories have given birth to some high activity profile drugs 7. Indeed, the market and public demand have been so great that there is a great risk that many medicinal plants today, face either extinction or loss of genetic diversity 8. Knowledge of the chemical constituents of the plant is desirable because such information will value for the synthesis of complex chemical substances.
Santalum rubrum, with the common names red sanders, red sandalwood, and saunders wood, is a species of Rubrum to the Southern Eastern Ghats mountain range of South India 9. This tree is valued for the rich red color of its wood. Demand for red sandalwood is mainly in the overseas market, said a trader and it comes mainly from countries like China, Japan, Myanmar and other others in East Asia. The red sandalwood has medical advantages. According to the Institute of Wood Science and Technology, the wood gives cooling effect when applied externally for inflammations, headache, bilious affections, and skin diseases and improves treating headache, skin diseases. This plant, S. rubrum, is widespread across Bangladesh, India, Malaysia, Canada, and Australia. Bangladesh is a good repository of medicinal plants belonging to various families. Herbal medicines have a strong traditional or conceptual base and the potential to be useful as drugs in terms of safety and effectiveness, leads for treating different diseases 10. Few interesting compounds could be found out through the investigation which may be unknown, pharmacology active and highly potent. Results obtained, if significant, can be used as a treatment option against some diseases. This may provide cost-effective treatment due to its availability in Bangladesh. Hence, the objective is to explore the possibility of developing new drug candidates from this plant for the treatment of various diseases.
Proximate analysis is a system of analysis of nutrients also termed “conventional analysis” in which the gross components (protein, fat, carbohydrate, ash) of the food material rather than individual nutrients (amino acid, fatty acid, mono-saccharides) are determined 11.
However many researchers have been carried out on Santalum rubrum, is but no systematic research on the fatty acid composition of seeds of the plant by GC-MS analysis has been reported. Therefore, the present study was undertaken to carry out a complete investigation of the compositions of fatty acids from the seeds of from petroleum ether extract of Santalum rubrum with GC-MS analysis.
MATERIALS AND METHODS:
Collection of Plant Material: Fully matured fresh seeds of Santalum rubrum were collected from the local area of Rajshahi district, Bangladesh in April 2016 and identified by the taxonomist of Bangladesh National Herbarium, Dhaka, where a voucher specimen (no. = 43205) has been deposited.
Preparation of Sample: The matured seeds were washed to remove dirt. Then it was oven-dried at a reduced temperature of less than 45 ºC to make it suitable for grinding purpose. The screened (20 mesh) powder was then stored in an air-tight container with marking for a future experiment.
Solvents and Chemicals: Petroleum ether (b.p 40-60 ºC, Merck, Germany) and other chemicals of AR grade, under normal atmospheric pressure were employed for the extraction of plant material. Solvent from extract was recovered under distillation, and the dried extracts were preserved in a refrigerator.
Physico - Chemical Studies: Physico-chemical characteristics of Santalum rubrum seed oil such as Specific gravity, iodine value (pet-ether extract), acid value, ester value, saponification value, peroxide value, color, solubility, were determined by following the standard procedures 12, 13, and the results were shown in Table 1.
Extraction of Fatty Acids and Preparation of Methyl Ester (FAMEs): The matured seeds of Santalum rubrum was collected and washed individually from running tap water to remove soil particles and other dust. Then they were dried at room temperature and powdered by Fritsch mortar grinder, Germany. The natural fatty acids were extracted separately from the seed powder (100 gm) of the plant with petroleum ether (b.p 40 ºC -60 ºC) in a Soxhlet apparatus for 72 h.
The extracts were concentrated under reduced pressure in a rotary evaporator. The extracts were filtered using Whatman no.1 filter paper and then vacuum distilled to remove the solvent completely. The extracts from the seeds of Santalum rubrum was 6.38 gm (6.38% w/w) Petroleum ether extracts for seeds of Santalum rubrum were kept in a nitrogen atmosphere in a refrigerator. The fatty acids present in the extracts were converted to Fatty Acid Methyl Esters (FAMEs) first and analyzed according to the method reported by Griffin 14 for GC-MS analysis.
The fatty acid composition was determined by analysis of their methyl esters. The Fatty Acid Methyl Esters (FAMEs) were prepared by esterification reaction by using BF3-MeOH complex according to AOAC method 15. 10 mg of extract of seeds was taken in a screw-capped glass tube. 1 ml of BF3-MeOH complex were added and then heated at 100 ºC for 1h in a water bath. After that, it was cooled at room temperature, and 1ml of deionized water and 2 ml of hexane were added. The glass tube was vortexed and centrifuged at low RPM for two min. The upper layer was collected using a syringe and kept in a closely tight glass vial in the refrigerator. Then the prepared FAMEs were ready to analyze.
Gas Chromatograph-Mass Spectrum Analysis: GC-MS analysis of seeds of Santalum rubrum from petroleum ether extracts were carried out on an Agilent 7890A system equipped with Mass Spectrophotometer detector and splitless injection system. The GC was fitted with an HP-5MS capillary column (30 m × 0.25mm: film thickness: 0.25μm). The temperature program was as follows: injector temperature 260 ºC, initial oven temperature at 70 ºC, then increased at 10 ºC/min to 150 ºC for 5 min., then 12 ºC/min to 200 ºC, for 15 min. and then 12 ºC/min to 220 ºC, for 15 min. Helium was used as the carrier gas at 17.69 psi pressure with a flow of 0.6 ml/min. Samples were dissolved in methanol, and 1 μl aliquot was injected automatically. MS was set in scan mode. The ionization was electron ionization. The mass range was set in the range of 50-550 m/z. MS spectra of separated components were identified on NIST libraries for fatty acid compositions.
RESULTS AND DISCUSSION: The result of the Physico-chemical properties of Santalum rubrum seed fatty oil is appeared in Table 1.
TABLE 1: PHYSICO-CHEMICAL PROPERTIES OF FATTY OIL OF SANTALUM RUBRUM SEED FATTY OIL
| Physical properties | Santalum rubrum seed fatty oil | ||
| Oil yield | 6.38% | ||
| Organoleptic | Taste | Spicy, bitter taste | |
| Odor | Spicy | ||
| Color | Orange-yellow | ||
| Appearance at room temperature (30 ºC) | Homogeneous, opaque liquid, lighter than water | ||
| Specific gravity at 30 ºC | 0.938 ± 0.037 | ||
| Acid value (mg KOH/g) | 8.80 ± 0.50 | ||
| Saponification value (mg KOH/g) | 68.14 ± 0.30 | ||
| Ester value | 13.4 | ||
| Iodine value (Hanus method) | 78.73 ± 0.99 | ||
| Peroxide value | 28.93-32.40 | ||
| Unsaponifiable matter (%) | 0.78 ± 0.35 | ||
|
Solubility in |
Alcohol | Soluble | |
| Distilled water | Insoluble | ||
| Chloroform | Soluble | ||
| CCl4 | Soluble | ||
| Pet-ether | Soluble | ||
| Diethyl ether | Soluble | ||
| n-hexane | Insoluble | ||
Properties of the oil such as acid value, iodine value, and saponification value usually give the structural, stability and quality information about the oils. The saponification value is the conversion of glycerol or ester into soap. The term is often used to describe the hydrolysis of an ester by alkali. The amount of alkali required to saponify a given amount of oil depends upon the molecular weight of the fatty acid present in the oil or fat 16.
Saponification value tells whether the oil or fat contains a lower proportion of higher fatty acids and also whether it contains a high proportion of lower fatty acids or higher fatty acids. Saponification value was found 68.14 mg, KOH/g, Table 1, indicating the presence of moderately higher molecular weight fatty acids in oils. High molecular weight fatty acids are not good for human health. In that sense, fats or oils having low saponification number should be preferred 17, representing typical C16 and C18 oils.
Iodine value is expressed in gm of iodine absorbed by 100 gm of oil or fat. It indicates the degree of unsaturation of the constituent oil and is thus a relative measure of the unsaturated bonds present in the oil 16. The iodine value is the characteristic of oil. One double bond absorbs one molecule of iodine. Unsaturated compounds absorb iodine (in soluble form) and form satura 78.73 which were almost similar to those for olive oil (76-90) 16, 17, 18, 19, 20. Iodine value increases as the degree of unsaturation increases.
So, the results indicate that the oil possesses a high degree of unsaturation. Therefore the extracted fatty oil possesses a high proportion of higher unsaturated fatty acid. Acid value denotes the number of mg of potassium hydroxide (KOH) needed to neutralize the fatty acid in the oil or fat. The free fatty acid is produced by the hydrolytic decomposition of the oil. The low acid value is an indication of the freshness of the oil. Acid values of S. rubrum seed oil were found to vary from (8.80).
Peroxide value is the number of mili-equivalent of active oxygen that expresses the amount of peroxide contained in 1000 g of the substance or peroxide oxygen per 1 kilogram of fat or oil. This value is a measurement of the extent to which rancidity reactions have occurred during storage. The double bonds found in fats or oils play a role in autoxidation. Oils with a high degree of unsaturation are most susceptible to autoxidation. The best test for autoxidation (oxidative rancidity) is the determination of the peroxide value.
Autoxidation is a free radical reaction involving oxygen that leads to deterioration of fats and oils which form off flavors and off-order. The deteriorated oil is hazardous for human health, e.g. may cause of heart disease and also promote the platelet stickiness that leads to blood clot formation and may cause stomach problem etc. 21. Peroxide values of Santalum rubrum seed oil were found within (28.93-32.40). Peroxide value showed that the oil had much free active oxygen enabling autoxidation of the oil 22.
Unsaponified matter contents (0.78-0.8) which is very much closer to some edible oils such as soybean oil (0.2-1.5)%, sunflower oil ( 0.3-1.5) %, mustard oil (0.9-1.0)%, and olive oil (0.5-1.5)%. Unsaponified matter includes those substances frequently found dissolved in fats and oils which cannot be saponified by caustic alkali but are soluble in ordinary fat solvents. This proportion varies between (0.5-2.0)%. So in this respect, the Santalum rubrum seed oil can be treated as edible oil 16, 19, 20.
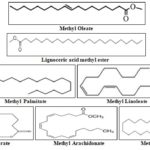
The GC-MS analysis of fatty acids of seeds of Santalum rubrum petroleum ether extract showed the presence of 7 compounds for seeds of Santalum rubrum. GC analyzed results which include the active principles with their retention time, molecular formula, molecular weight and composition of the fatty acids of seeds of Santalum rubrum from petroleum ether extract are presented in Table 2. Total 7 fatty acids were identified as their methyl esters in the case of seeds of Santalum rubrum. The major constituent was methyl linoleate (38.44%) with retention time 16.07.
FIG. 1: STRUCTURE OF THE IDENTIFIED FATTY ACID ESTERS FROM GC-MS ANALYSIS OF PETROLEUM ETHER EXTRACT OF SEEDS OF SANTALUM RUBRUM
TABLE 2: GC-MS ANALYSIS OF FATTY ACIDS FROM PETROLEUM ETHER EXTRACT OF SEEDS OF S. RUBRUM
| S. no | Retention time (min) | Name of the compound | Molecular weight | Molecular Formula | Conc. (%) |
| 1 | 13.60 | Methyl Palmitate | 270.45 | C17H34O2 | 33.18 |
| 2 | 16.44 | Methyl Stearate | 298.50 | C19H38O2 | 4.80 |
| 3 | 16.13 | Methyl Oleate | 296.49 | C19H36O2 | 11.35 |
| 4 | 16.07 | Methyl Linoleate | 294.47 | C19H34O2 | 50.65 |
| 5 | 18.75 | Methyl Arachidonate | 318.50 | C21H34O2 | 3.50 |
| 6 | 21.50 | Methyl Behenate | 354.61 | C23H46O2 | 2.72 |
| 7 | 23.96 | Lignoceric acid methyl ester | 382.66 | C25H50O2 | 29.55 |
CONCLUSION: The present study found 7 constituents from seeds of petroleum ether extract of Santalum rubrum by Gas Chromatography-Mass Spectroscopy (GC-MS) analysis. The presence of these chemical compounds justified the extensive uses of seeds of the plant by a traditional practitioner to treat various ailments. It could be concluded that S. rubrum contains various chemical constituents that can be bioactive compounds of medical importance. However, further studies are needed to evaluate its bioactivity & toxicity profile.
Acknowledgement: We are grateful to the Chemistry Department, Dhaka University for allowing us to do an analysis of the petroleum ether extract of plant materials. We are also thankful to the Director, BCSIR Laboratories, Dhaka, Dr. Sarwar Jahan, for providing necessary facilities to carry out this research work.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Cragg GM, Newman DJ: natural Product drug discovery in the next millennium. Pharm Biol 2001; 1: 8-17.
- Sofowora A: Medicinal plants and traditional medicine in Africa: Spectrum Books Ltd, Ibadan, Nigeria 1993: 289.
- Mensah JK, Okoli RI, Ohaju-Obodo JO and Eifediyl K: Phytochemical, Nutritional and medical properties of some leafy vegetables consumed by Edo people of Nigeria. African Journal of Biotechnology 2008; 7(14): 2304-2309.
- Gill LS: Ethnobotanical uses of plants in Nigeria: University of Benin Press, Benin city 1992; 350.
- Banso A and Adeyemo SO: Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. African Journal of Biotechnology 2007; 6: 1785-1787.
- Ivanova D, Gerova D, Chervenkov T and Yankova T: Polyphenol and antioxidant capacity of Bulgarian medicinal plants. J Ethnopharmacol 2005; 96: 145-150.
- Mandal V, Mohan Y and Hemalatha S: Microwave assisted extraction-An innovative and promissing extraction tool for medicinal plant research. Pharmacog Rev 2007; 1: 7-18.
- Misra A: Studies on biochemical and physiological aspects in relation to phytomedicinal qualities and efficacy of the active ingredients during the handling, cultivation and harvesting of the medicinal plants. Journal of Medicinal plants research 2009; 3: 1140-1146.
- Brown H, Stephen S: Red Silk-Cotton, Red Cotton Tree, Kapok. Gardening Publications A-Z, University of Florida, 2011.
- Rahman, AHMM and Khanom A: Taxonomic and ethno medicinal study of species from Moraceae (mulberry) family in Bangladesh flora. Research in Plant Sciences, USA, 2013; 1(3): 53-57.
- Prohp TP, Ihimire IG, Madusha AO, Okpala HO, Erevor JO and Oyinbo CA: Some Anti-Nutritional and mineral contents of extra-cotyledonous deposit of pride of Barbados ( pulcherrima). Pakistan Journal of Nutrition 2006; 5: 114-116.
- Mowlah G, Sheikh NM and Kamal ASM: A Hand Book on Edible Oils Fats, with special reference to Bangladesh, City press, Dhaka - 1000, Bangladesh, Edition 1st, 1990: 44.
- Morshed S, Alam MK, Begum A, Shariar SMS, Sharmin KN and Akhter S: Physiochemical properties and Chemical constituents of oil from Joan Seed ( ammm L). Journal of Environ Sci & Natural Resources 2012; 5(2): 15-21.
- Association of Official Analytical Chemists: “Official Methods of Analysis”. Washington DC. Edition 16th, 1995: Chapter -41: 14-15.
- AOAC: Association of Official Analytical Chemist. Official Methods of Analysis, Official methods 28.059. Sydney, Williams. Arlington, USA, Edition 14th, 1984.
- Mowla G, Sheick NM and Kamal ASM: Hand Book on Edible Oils and Fats with Special Reference to Bangladesh, Edition 1st, (University of Dhaka, Dhaka, Bangladesh). 1990: 9-172.
- Meyer LH: Food Chemistry. CBS Publ. and Distr., Delhi, India. Edition 1st, 1987: 12-64.
- Ching KC: Fatty acid in foods and their health Implications. Marcel Dekker Inc. Publisher, New York. Edition 2nd, 2000: 209-238.
- Jacobs MB: The Chemical Analysis of Food Products. CBS Publ. Edition 3rd, 2006: 365-383.
- Nollet LM: Handbook of Food analysis, Physical Characterization and Nutrient Analysis (Food Science and Technology). Marcel Dekker Inc. Publishers, New York, USA. Edition 2nd, Vol. 1, 2004: 221-274.
- Richard D, Brien. O’, Lynn A, Jones C, Clay King, Philip J, Wakelyn, Peter J and Wan: Bailey’s Industrial oil and fat products, Edition 6th, Vol. 1, 2005: 173-274.
- Lange NA: Handbook of Chemistry, Hand book Publishers Inc., Sandusky, Ohio. Edition 14th, 1944: 678.
How to cite this article:
Aziz S, Mitu TK and Al-Reza SM: Chemical composition of fixed oil from the seeds of Santalum rubrum by GC-MS. Int J Pharmacognosy 2018; 5(8): 517-21. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(8).517-21.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.