CAFFEIC ACID ESTERS: SYNTHETIC METHODOLOGIES, ANTICANCER AND ANTI-INFLAMMATORY ACTIVITIES
HTML Full TextCAFFEIC ACID ESTERS: SYNTHETIC METHODOLOGIES, ANTICANCER AND ANTI-INFLAMMATORY ACTIVITIES
Ayele Wondatir
Department of Chemistry, College of Natural Science, Arba Minch University, Arba Minch, Ethiopia.
ABSTRACT: Hydroxycinnamic acids (HCA) and derivatives are well-known phenolic compounds ubiquitous in plants, showing relevant antioxidant properties as well as cytotoxicity toward several tumor cell lines. They can inhibit cell growth in a manner strongly dependent on their structural properties. Caffeic acid (CA) is one of the most widely distributed hydroxycinnamate and phenylpropanoid metabolites in plant tissues and agricultural wastes. Caffeic acid and its esters have a variety of biological activities, including antimicrobial, antioxidant, anti-inflammatory, anticancer, antiviral, anti-diabetic, anti-HIV, anti-influenza and antimalarial activities. In most cases, the synthesis of alkyl esters can significantly improve their function. This review is an overview of the available information about the chemical synthesis, anticancer and anti-inflammatory activities of caffeic acid esters. Considering the relevance of these compounds in human health, many of them have been the focus of reviews, taking as a center their obtaining from the plants. There are few revisions that compile the chemical synthesis methods, in this way, we consider that this review does an important contribution.
Keywords: Caffeic acid, Caffeic acid esters, Anticancer, Anti-inflammatory
INTRODUCTION: Hydroxycinnamic acids (HCA) and derivatives are well-known phenolic compounds ubiquitous in plants, showing relevant antioxidant properties as well as cytotoxicity toward several tumor cell lines. They are able to inhibit cell growth in a manner strongly dependent on their structural properties 1. Caffeic acid 1(CA; 3,4-dihydroxycinnamic acid) Fig. 1 is one of the most widely distributed hydroxycinnamate and phenylpropanoid metabolites in plant tissues and agricultural wastes 2. CA, as a natural antioxidant, has received increasing attention with regard to its applications in the food, health, cosmetic and pharmaceutical industries because of its numerous biological activities, such as anti-mutagenic, anti-proliferative and anti-oxidant activities 3.
Moreover, it has been proved in many biological investigations that caffeic acid and its analogues also display anti-inflammatory 4, antimicrobial 5, anti-HIV 6, anti-influenza 7, antidiabetic 8 and antimalarial 9 activities. However, CA exhibits low solubility and stability in various solvent systems, thus it is necessary to enhance its practical applicability by improving its solubility 10.
The strategy of esterification of hydrophilic CA with lipophilic molecules, such as aliphatic alcohols, could be employed to alter its solubility in hydrophobic media 11. In addition, the synthesis of alkyl esters can significantly improve their function. For example, it was found that alkyl esters of CA have a higher antioxidant activity and lipophilicity than CA to protect neuronal PC12 cells against oxidative stress 12. Therefore, it is advantageous to synthesizе alkyl esters of caffeic acid based on both their biological function and potential application. There are many literature reports that address the different caffeate biological activities, much research remains to be done on this family of polyphenols, and new derivatives with potentially higher activity than natural or synthetic products reported can be obtained. This review intends to summarize the several synthetic methods, anticancer, and anti-inflammatory activities of these caffeic acid esters.
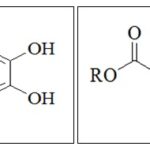
FIG. 1: CHEMICAL STRUCTURE OF CAFFEIC ACID AND CAFFEIN ACID ESTER (R= ALKYL GROUPS)
Chemical Synthesis of Caffeic Acid Esters: Caffeic acid esters may be obtained through organic synthesis methodologies from caffeic acid itself or from other chemical precursors. In different reports, caffeic acid esters have been synthesized in different methods.
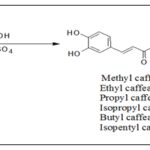
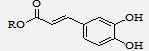
Direct Esterification: Caffeic acid alkyl esters can be obtained by many different pathways. Direct esterification (Fisher method) is one of the most used synthetic strategies to obtain esters with a short alkyl chain, using in most of the cases sulfuric acid as catalyst 13-16. Sanderson et al. 17 obtained caffeic acid esters (methyl caffeate 2, ethyl caffeate 3, n-propyl caffeate 4, Isoproyl caffeate 5, butyl caffeate 6, and isopentyl caffeate 7) by refluxing aliphatic alcohol, caffeic acid, and sulfuric acid, for 2 hours Scheme 1.
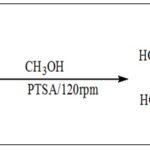
Wang et al. 18 also synthesized methyl caffeate 2 using p-toluenesulfonic acid (PTSA) by the Fisher method Scheme 2.
SCHEME 1: SYNTHESIS OF CAFFEIC ACID ESTERS USING H2SO4
SCHEME 2: SYNTHESIS OF CAFFEIC ACID ESTERS USING PTSA
The Acyl Chloride Method: One of the most common methods reported in the literature for the synthesis of caffeic acid esters uses thionyl chloride as a reagent and transforms caffeic acid in caffeoyl chloride 19-21. Chou et al., 22 obtained caffeic acid esters from Caffeic acid and alcohols in the presence of SOCl2 Scheme 3.
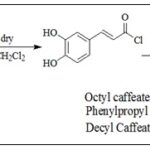
SCHEME 3: SYNTHESIS OF CAFFEIC ACID ESTERS USING ACYL HALIDE
Reaction of Caffeic Acid and Halo Hydrocarbons: Some authors synthesized alkyl caffeates by nucleophilic displacement of a halogen atom from an alkyl halide in a basic medium from caffeic acid Scheme 4 23.
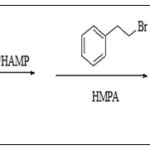
SCHEME 4: SYNTHESIS OF CAPE FROM HALOGEN SUBSTITUTED HYDROCARBON
Wittig Reaction: Wittig reaction can be used to obtain esters. The most commonly used reagents are esters of α-haloacetic acid, which, by reaction with triphenylphosphine, produce the corresponding phosphonium salt. This phosphonium salt further reacts with benzoic acid in appropriate condition produce t-butylcaffeate Scheme 5 23, 24.
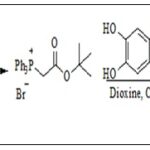
SCHEME 5: SYNTHESIS T-BUTYL CAFFEATEVIA WITTIG REACTION
Malonic Acid Monoester Method: Malonic acid monoester method is another convenient method for the synthesis of caffeic acid esters. Firstly, the malonate di-esters prepared using Wakasugi method.
The malonic acid mono-esters obtained by saponification of the malonate di-esters in the presence of one equivalent of potassium hydroxide. Finally, by condensation reaction, prepared caffeic acid esters Scheme 6 25.
SCHEME 6: SYNTHESIS OF HEPTYL CAFFEATE FROM MALONIC ACID MONOESTER
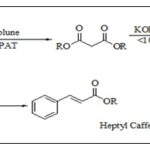
Researchers synthesized CAPE using toluene as solvent, catalyst DPAT catalyzed malonic acid, and esterified for malonic acid diester. It reacted equimolarly with KOH for malonic acid monoester potassium single. The malonic acid monoester was obtained by acidification, then condensate for CAPE with 3,4-dihydroxy benzaldehyde by Knoevenagel-Doebner Scheme 7 26.
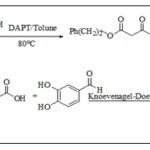
SCHEME 7: SYNTHESIS OF CAPE FROM MALONIC ACID MONOESTER
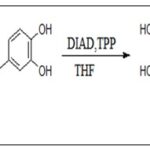
Mitsunobu Esterification of Caffeic Acid: Mitsunobu reaction is used in the synthesis of caffeic acid esters 27–29. Chapado et al., 28 used this method (triphenylphosphine (TPP) and diisopropyl azodicarboxylate (DIAD) in dry tetrahydrofuran as solvent at room temperature) to obtaindihydroxyphenethyl caffeate 14 Scheme 8.
SCHEME 8: SYNTHESIS OF DIHYDROXY PHENETHYL CAFFEATE USING MITSUNOBU ESTERIFICATION
Enzymatic Synthesis of Caffeic Acid Esters: Pang et al. 30 was synthesized propyl caffeate using lipase-catalyzed transesterification in ionic liquid. Propyl caffeate was obtained by transesterification reaction in a 5 mL screw-capped vial at a certain temperature for 38 h with a constant stirring speed of 120 rpm. Methyl caffeate 2or ethyl caffeate3 was dissolved into 1 mL ionic liquid. The reaction was initiated by adding immobilized lipase and 1-propanol. The maximum propyl caffeate4 yield of 98.5% was obtained using lipase-catalyzed transesterification using Novozym 435 as a biocatalyst, [Bmim] [CF3SO3] as a medium, a molar ratio of methyl caffeate to 1-propanol of 1:5, a mass ratio of methyl caffeate to lipase of 1:20, and a reaction temperature of 60 ºC. The two-step conversion of caffeic acid to propyl caffeate via methyl caffeate is an efficient way to prepare propyl caffeate with an overall yield of 82.7% Scheme 9.
Wang et al., 31 improved this method in the microreactor in a short period of time (2.5 h) with a flow rate is 2 µL/min which kinetic constant, Km, was 16 times lower than that of a batch reactor.
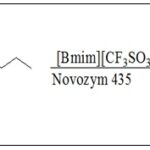
SCHEME 9: SYNTHESIS OF PROPYL CAFFEATE USING TRANSESTERIFICATION
Researchers reported candida antarctica lipase B (Novozym435) had the scope to generate CAPE by catalytic esterification of caffeic acid and phenethyl alcohol Scheme 10 31, 33.
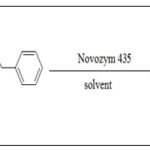
SCHEME 10: SYNTHESIS OF CAPE USING NOVOZYM 435
Chyba et. al., 34 reported the enzymatic caffeoylation of methyl β-D-glucopyranoside using vinyl and 2,2,2-trifluoroethyl caffeates as caffeoyl donors and lipase from thermomyces lanuginosus (Lipozyme TL IM) Scheme 11.
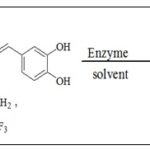
SCHEME 11: SYNTHESIS OF METHYL Β-D-GLUCOPYRANOSIDE USING ENZYME
Biological Activities of Caffeic Acid Esters:
Anticancer Activity: Cancer is one of the world’s major risks to human health. Epidemiological studies had shown that eating various rich vegetables, fruit, and other natural foods could significantly reduce the risk of cancer in humans 35. CAPE was subsequently considered to be a potent anticancer component of propolis 36, and it was a natural anticancer drug with a good curative effect and with little side effect 37.
CAPE was an earlier confirmed antitumor component against general in vivo and in vitro neoplasm models, melanomas, lung and prostate cancers, and so on 38.
Research showed that CAPE in propolis had a great impact on melanoma, colorectal and gastric cancer cell lines 39. CAPE exhibited inhibitory effects on the motility and invasion of C6 glioma cells when tested with scratch assay and Boyden chamber assay.
Furthermore, CAPE induced the expression of nerve growth factor and p75 neurotrophin receptor, which were involved in neuroal cell differentiation, inhibited the activity of MMPs, and induced the expression of RhoB, a tumor suppressor showing CAPE as an agent that possessed antitumor progression potential 40.
The effect of CAPE on cholangiocarcinoma (CCH) growth both in vitro and in-vivo was also studied. It decreased the growth of a number of CCH cells but not of normal cholangiocytes.
On the other hand, Bax expression was increased, whereas Bcl-2 expression was decreased in cells treated with CAPE. In BALB/c nude mice implanted subcutaneously with MzChA-1 cells treated with daily CAPE for 77 days, tumor growth was decreased, and tumor latency was increased two-fold 41.
Chiang found CAPE could significantly inhibit the growth of colorectal tumors in a mouse xenograft model. The mechanisms of action included modulation of PI3-K/Akt, AMPK, and m-TOR signaling cascades both in vitro and in-vivo 42.
Concerning various combined studies (both in-vivo and in-vitro), Chen found the effects of CAPE on tumor growth in relation to IL-1β signaling when examined in CE81T (human esophageal cancer cells) and CCL-241 cells (human normal intestine cell line) 43.
The effects of CAPE in a breast cancer model, including tumor growth both in-vitro and in-vivo, were examined, and its effects on the cell cycle, apoptosis, and angiogenesis in the hormone receptor-negative MDA-231 and hormone receptor-positive MCF-7 breast cancer cell lines were analyzed 44. Sanderson et al., 17 synthesized and evaluated the effects of caffeic acid1, CAPE 11, and synthetic alkyl caffeic acid esters (methyl caffeate 2, Ethyl caffeate 3, propyl caffeate 4, isopropyl caffeate 5, n-butyl caffeate 6, isopentyl caffeate 7) Table 1 on cell viability and androgen-dependent cell proliferation, subcellular localization and expression of androgen receptor (AR) and secretion of prostate-specific antigen (PSA) in LNCaP human hormone-dependent prostate cancer cells.
Fiuza et al., 14 also tested caffeic acid esters (methyl caffeate 2, propyl caffeate 4, octyl caffeate, 8), in a human cervix adenocarcinoma cell line-HeLa (epithelial-like adherent line).
In order to determine the degree of toxicity of these compounds towards healthy cells, experiments were also carried out in non-neoplastic cells-fibroblasts from human embryonic lung tissue (L-132).
Nagaoka et al., 45 synthesized caffeic acid esters and were tested for their anti-proliferative activities toward six different tumor celllines, that is, murine colon 26-L5 carcinoma (colon 26-L5), murine B16-BL6 malonoma (B16-BL6), murine Lewis lung carcinoma (LLC), human HT-1080 fibrosarcoma (HT-1080), human lung A549 adenocarcinoma (A549), and human cervix HeLa adenocarcinoma (HeLa) cell lines. The anti-proliferative activities of 11,18–26 and 22-29are summarized in terms of their EC50 values in Table 1.
All these esters showed stronger anti-proliferative activity than caffeic acid, and all the compounds except for20 and 21 showed the strongest activity toward colon26-L5 cell line.
Interestingly, the CAPE analogues revealed no cytotoxic effect toward primary cultured mouse hepatocytes up to 100 mM concentration.
This indicates that CAPE and its analogues possessed selective anti-proliferative activity toward colon 26-L5 cellline. Especially, the activities of compounds 20 and 26 (EC50: 0.02 and 0.02µM, respectively) were stronger than those of 5-fluorouracil (EC50:0.06 µM) and doxorubicin (EC50: 0.04 µM), which were used as positive controls.
TABLE 1: CYTOTOXIC AND ANTI-PROLIFERATIVE ACTIVITIES OF CAFFEIC ACID AND CAFFEIC ACID ESTERS AGAINST DIFFERENT CANCER CELL LINES.
Heterocyclic esters of caffeic acid were synthesized using Mitsunobu reaction. Those heterocyclic esters of caffeic acid 30-34 Table 1 were evaluated for their cytotoxic activity against HeLa, SK-OV-3, and HT-29 cancer cell lines. All compounds had good inhibitory activity (IC50 = 10-200 μM) against HeLa and HT-29 but they did not show significant inhibitory activity (IC50 > 100 μM) against SK-OV-3 29.
Anti-Inflammatory Activities: Caffeic acid has been shown to possess anti-inflammatory properties, since it inhibits 5- and 12 lipoxygenase activities, 46 in addition to inhibiting PKC, PKA and NF-kB activation induced by ceramides in U937 cells 47. These anti-inflammatory actions extend to some of the caffeic acid derivatives.
Caffeic acid phenetyl ester (CAPE), a caffeic acid derivative originally isolated from the honeybee propolis, is able to specifically and potently inhibit NF-kB 48, 49. Furthermore, CAPE is effective in suppressing 5-LOX activity, 50 as well as TPA-induced PGE2 production in human oral epithelial cells 51. Incubation of RAW macrophages with this caffeic acid derivative inhibits LPS-induced iNOS expression 52. Finally, in a rat model of vascular injury, the administration of CAPE diminishes COX-2 expression, NF-kB activation, and restenosis 53.
Da Cunha et al., 54 studied in vitro and in vivo effects of five caffeic acid derivatives (caffeic acid, methyl, ethyl, butyl, octyl and benzyl esters) and compared their actions to those of CAPE.
In the model of LPS-induced nitric oxide (NO) production in RAW 264.7 macrophages, the pre-incubation of all derivatives inhibited nitrite accumulation on the supernatant of stimulated cells, with mean IC50 (µM) values of 21.0, 12.0, 8.4, 2.4, 10.7 and 4.80 for methyl caffeate 2, ethyl caffeate 3, butyl caffeate 6, octyl caffeate 9, benzyl caffeate18and CAPE11, respectively (Table-3).
The effects of caffeic acid derivatives seem to be related to the scavenging of NO, as the compounds prevented SNAP-derived nitrite accumulation and decreased iNOS expression. Uwai et al., 19 examined the function of the ester functional group and the alkyl side chain (alcoholic part) and transformed caffeic acid to several derivatives. The inhibitory effect of these derivatives on NO production in murine macrophage RAW264. When the alkyl chain was C0–C11, the EC50 values of the caffeic acid esters decreased with the increasing length of the alkyl chain. In contrast, for esters with C6–C18 alkyl chains, their EC50 values were almost constant. Undecyl 57 and dodecyl 27 caffeate were most potent cytotoxic compounds Table 2. Isopropyl ester 5, with the same alkyl chain length as ethyl ester 3 and the same carbon number as propyl ester 4, showed a similar EC50 value to propyl ester 4.
A similar result was obtained in the case of Cyclohexyl 40 and benzyl 18 esters showed low cytotoxicity. These results suggested that the balance between lipophilicity and the size of the alcoholic moieties affected the cytotoxicity levels. However, prenyl 41, geranyl 42, and farnesyl 43 esters, which were anticipated to be lipophilic and cell membrane-philic relative to their corresponding straight chain esters, showed EC50 values 2 to 6 times higher than butyl 6, octyl 8, and dodecyl 27 caffeates, respectively.
TABLE 2: TOXICITY AND NO INHIBITION OF THE CAFFEIC ACID AND CAFFEIC ACID ESTERS
CONCLUSION: In addition to extraction from natural sources, there are cheap and easy to make synthetic methods for obtaining caffeic acid esters. These methods, unlike the extractive ones, could provide enough quantity of caffeic acid derivatives for their multiple uses, besides guaranteeing the preservation of the plants as a natural resource. In this review, the alternatives for the synthetic obtaining of esters of caffeic acid by simple synthetic methods and their anticancer and anti-inflammatory activities are shown.
ACKNOWLEDGMENT: Nil
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES:
- Gomes CA, da Cruz, TG, Andrade JL, Milhazes N., Borges F and Marques MP: Anticancer activity of phenolic acids of natural or synthetic origin: a structure−activity study.J Med Chem 2003; 46: 5395-01.
- Wang J, Lu D, Zhao H, Ling X, Jiang B and Ouyang P: Application of response surface methodology optimization for the production of caffeic acid from tobacco waste. Afr J Biotech 2009; 8: 1416.
- Tsai Y, Chiou S, Chan K, Sung J and Lin S: Caffeic acid derivatives, total phenols, antioxidant and antimutagenic activities of echinacea purpurea flower extracts.LWT – Food Sci Technol 2012; 46: 169.
- Kyung-Min S, In-Tae, K, Young-Mi P, Joohun H, Jong-Won C, Hee-Juhn P, Yong SL and Kyung-Tae L: Anti-inflammatory effect of caffeic acid methyl ester and its mode of action through the inhibition of prostaglandin E2, nitric oxide and tumor necrosis factor-α production. Biochem Pharmacol 2004; 68 (12): 2327-36.
- Roman M, Iveta H, Vladimír F and Jan Š: Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J Food Sci 2010; 28(4): 275-79.
- Fabrice B and Philippe C: Anti-HIV activities of natural antioxidant caffeic acid derivatives: toward an antiviral supplementation diet. Curr Med Chem 2005; 12; 1811-18.
- Yuanchao X, Bing, H, Kexiang Y, Fangyuan S, Tianqi L and Wenfang X: Caffeic acid derivatives: A new type of influenza neuraminidase inhibitors. Bioorg Med Chem Lett 2013; 23: 3556-60.
- Ganiyu O, Odunayo MA, Stephen AA, Ayodele JA and Adedayo OA: Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): a comparative study. J Basic Clin Physiol Pharmacol 2015; 26(2): 165-70.
- Sylvain GA, Olivia J, Ewa C, Hajatiana R, Herintsoa R, Gilles D, Pierre F and Michel F: In-vitro and in-vivo antimalarial activity of caffeic acid and some of its derivatives. J pharm Pharmacol 2018; 70: 1349-56.
- Aytekin AO, Morimura S and Kida K: Synthesis of chitosan-caffeic acid derivatives and evaluation of their antioxidant activities. J Biosci Bioeng 2011; 111: 212.
- Chen H Twu C, Chang J, Liu Y and Shieh C: Optimizedsynthesis of lipase-catalyzed octyl caffeate by Novozym®.Ind. Crop Prod 2010; 32: 522.
- Gu S, Wang J, Pan F, Pang N andWu F: A study of the esterification of caffeic acid esters. Acta Crystallogr 2012; E 68: O557.
- Lijy J, Vijay DG, Chaitaly TC, Amod VT, Jagannath JK and Satish MB: Methyl Caffeate Ether Derivatives as Future Potential Drug. J Chem Bio Phy Sci Sec A, 2014; 4: 139-46.
- Fiuza SM, Gomes C, Teixeira LJ, Girão Da Cruz, MT, and Cordeiro MNDS, Milhazes N, Borges F and Marques MPM: Phenolic acid derivatives with potential anticancer properties-A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg Med Chem 2004; 12: 3581.
- De Farias MO, Lima TC, Pérez ALAL, silva RHN, Oliveira AJMS, Lima EO and De souse DP: Antifungal Activity of Ester Derivatives from Caffeic Acid against Candida Species. IJPPR Human2016; 7 (1): 151-94.
- Dietmar S, Flávio RDN, Stuart AR and Damião PDS: Trypanocidal and cysteine protease inhibitory activity of isopentyl caffeate is not linked in trypanosoma Brucei.Parasitol Res 2016; 115(11): 4397-03.
- Thomas S, Hélène C, Cody P, Grégoire L-C, Jacques J-F, Aurélie FP, Martin JGH, Marc ES and Mohamed T: Anti-proliferative, antiandrogenic and cytotoxic effects of novel caffeic acid derivatives in LNCaP human androgen dependent prostate cancer cells. Bioorg Med Chem 2013; 21(22): 7182-93.
- Jun W, Shuangshuang G, Na P, Fangqin W and Fuan W: A study of the esterification of caffeic acid with methanol using p-toluenesulfonic acid as a catalyst. J Serb Chem Soc2013; 78 (7): 1023-34.
- Koji U, Yuu O, Takuma I, Syu-ichi K, Mitsuhiro T and Masaaki I: Inhibitory effect of the alkyl side chain of caffeic acid analogues on lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophages. Bioorg Med Chem 2008; 16: 7795-03.
- Li SC, Li H, Zhang F, Li ZJ and Cui JR: Anticancer activities of substituted cinnamic acid phenethyl esters on human cancer cell lines. J Chin Pharm Sci 2003; 12(4): 184-187.
- Hao C, Xiaojing H, Shengtao X, Hao S, Pengfei Z, Yue H, Jieyun J, Yijun S, Bo J, Xiaoming W, Hequan Y and Jingyi X: Discovery of novel hybrids of diaryl-1,2,4-triazoles and caffeic acid as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for cancer therapy. Eur J Med Chem 2016; 108: 89-03.
- Dev-Aur C, Yueh-Hsiung K, Ming-S C, Yuan-Yen C, Yi-Chen, C, His-Lin C, Wei-Tang CF, and Chin-Lin, H: Caffeate derivatives induce apoptosis in COLO 205 human colorectal carcinoma cells through Fas- and mitochondria-mediated pathways. Food Chem 2012; 131: 1460-65.
- Beth E, Corwin H, Sanjay K and Dias S: Mechanism of Toxicity of Esters of Caffeic and DihydrocaffeicAcids. Bioorg Med Chem 2001; 9: 199-09.
- Keisuke T, Yasuyuki Y, Eisuke K, Shigeki K, Osamu I, Keizo H and Jun K: Methyl caffeate as an α-glucosidase inhibitor from Solanum torvum fruits and the activity of related compounds. Biosci Biotechnol Biochem 2010; 74 (4): 741-45.
- Chun-nian X, Wei-xiao H, Wei Z and Guo-hong W: Synthesis and Crystal Structure of Heptyl 3-(3,4-dihydroxyphenyl)-2-propenoate. J Chem Crystallogr 2008; 38: 583-86.
- Xia C, Hu W: Synthesis of caffeic acid esters. J Chem Res 2005; 5: 332-34.
- Daniela IB, Takao K, Vassya SB, Zornitsa GK and Makoto U: Synthesis of Some Phenylpropanoid Monoglycerides via the Mitsunobu Protocol. Molecules 2005; 10: 552–58.
- Laura C, Pablo JLP, Sofía S, Joaquín A, Juan AR and Ginés MS: Synthesis and evaluation of the platelet antiaggregant properties of phenolic antioxidants structurally related to rosmarinic acid. Bioorg Chem 2010; 38: 108-14.
- Shima HEK, Mohsen A, Mohsen V, Abbas S, Ebrahim A and Farzad K: Synthesis, evaluation of anticancer activity and qsar study of heterocyclic esters of caffeic acid. Iran J Pharm Res 2013; 12 (4): 705-719.
- Na P, Shuang-Shuang G, Jun W, Hong-Sheng C, Fang-Qin W, Xi L, Xing-Yu Z and Fu-An WA: Novel chemoenzymatic synthesis of propyl caffeate using lipase-catalyzed transesterification in ionic liquid. Bio res Tech 2013; 139: 337.
- Jun W, Shuang-Shuang G, Hong-Sheng C, Liu-Qing Y and Xiang-Yang W: Rapid synthesis of propyl caffeate in ionic liquid using a packed bed enzyme microreactor under continuous-flow conditions. Bior Tech 2013; 149: 367-74.
- Stevenson DE., Parkar SG, Zhang J, Stanley RA, Jensen DJ and Cooney JM: Combinatorial enzymic synthesis for functional testing of phenolicacid esters catalysed by Candida antarctica lipase B (Novozym 435®. Enzyme Microb Technol, 2006; 40: 1078.
- Widjaja A, Yeh T and Ju Y: Enzymatic synthesis of caffeic acid phenethyl ester. J Chin Inst Chem Eng 2008; 39: 413.
- Andrej C, Vladimír M and Mária M: Effective enzymatic caffeoylation of natural glucopyranosides. Bioorg Med Chem Lett 2016; 26: 1567-70.
- Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL and Bertaqnoli MM: Plant phenolics decrease intestinal tumors in an animalmodel of familial adenomatous polyposis. Carcinogenesis 2000; 21: 921.
- Akyol S, Ozturk G, Ginis Z, Armutcu F, Yiqitoqlu MR and Akyol O: In-vivo and in-vitro antineoplastic actions of caffeic acid phenethyl ester (CAPE): Therapeutic perspectives. Nutr Cancer 2013; 65: 515.
- Brumfitt W, Hamilton-Miller and MJ and Franklin I: Antibiotic activity of natural product: 1. Propolis. Microbios 1990; 62: 19-22.
- Tolba MF, Azab SS, Khalifa AE: Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotcvtive and cardioprotective. IUBMB Life 2013; 65: 699.
- Eisinger M, Marko O, Ogata S and Old LJ: Growth regulation of human melanocytes mitogenic factor in extracts of melanomn, astrocytoma, and fibrolast cell lines. Science1985; 229: 984.
- Lin WL, Liang WH, Lee YJ, Chung SK and Tseng TH: Antitumor progression potential of caffeic acid phenethyl ester involving p75 (NTR) in C6 glioma cells. Chem Biol Interact 2010; 188: 607.
- Onori P, DeMorrow S, Gaudio E, Franchitto A, Mancinelli R, Venter J, Kopriva S, Ueno Y, Alvaro, D and Savage, J: Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-κB and induction of apoptosis.Int J Cancer 2009; 125: 565.
- Chiang EP, Tsai SY, Kuo YH, Pai MH, Liu HL, Rodriguez RL and Tang FY: Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-K/Akt and AMPK signaling pathways. PLOS One2014; 9: e99631.
- Chen MF, Lu MS, Chen PT, Chen WC, Lin PY and Lee KD: Role of interleukin 1 beta in esophageal squamous cell carcinoma. J Mol Med 2012; 90: 89.
- Wu J, Omene C, Karkoszka J, Bosland M, Eckard J, Klein CB and Frenkel K: Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibitsa diversity of anti-tumor effects in pre-clinical models of human breast cancer.Cancer Lett 2011; 308: 43.
- Nagaoka T, Banskota AH, Tezuka Y, Saiki I and Kadada S: Selective anti-proliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg Med Chem 2002; 10: 3351.
- Koshihara Y, Neichi T, Murota S-I, Lao A-N, Fujimoto Y and Tatsuno T: Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Bioch Bio 1984; Acta792, 92–97.
- Nardini M, Scaccini C, Packer L and Virgili F: In vitro inhibition on the activity of phosphorylase kinase, protein kinase C and protein kinase A by caffeic acid and a procyanidin rich pine bark (Pinus marittima) extract.Biochim Biophys Acta1 2000; 474: 219-25.
- Alexandra S, Ceeneena UM, Franz JS, Sigrun E, Anton S and Reinhard G: Caffeic acid phenethyl ester protects against oxidative stressand dampens inflammation via hemeoxygenase 1. Inter J Oral Sci 2019; 11: 6.
- Natarajan K, Singh S, Terrence RBJ, Grunberger D and Aggarwal BB: Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kB. Proc Natl Acad Sci USA 1996; 93: 9090–95.
- Sudina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV and Varfolomeev SD: Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Letts1993; 329: 21 –24.
- Song YS, Park E-H, Hur GM, Ryu YS, Lee YS, Lee JY, Kim YM and Jin C: Caffeic acid phenethyl ester inhibits nitric oxide synthase gene expression and enzyme activity. Cancer Lett 2002; 175: 53–61.
- Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Kobolt C. Mestre JR, Grunberger D, Sacks PG, Tanabe T and Dannenberg AJ: Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res 1999; 59: 2347–52.
- Maffia P, Ianaro A, Pisano B, Borrelli F, Capasso F, Pinto A and Ialenti A: Beneficial effects of caffeic acid phenethyl ester in a rat model of vascular injury. Br J Pharmacol 2002; 136: 353-60.
- Fernanda MDC, Danielle D, Jamil A, Fatima CB, Rivaldo N, Maria MC and Joao BC: Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radic Res 2004; 38: 1241-53.
How to cite this article:
Wondatir A: Caffeic acid esters: synthetic methodologies, anticancer and anti-inflammatory activities. Int J Pharmacognosy 2020; 8(7): 266-77. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.8(7).266-77.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.