BOTANICAL STUDIES ON RAW HERBAL SAMPLES OF WOODFORDIA FRUTICOSA (L.) KURZ- AN IMPORTANT AYURVEDIC PLANT
HTML Full TextBOTANICAL STUDIES ON RAW HERBAL SAMPLES OF WOODFORDIA FRUTICOSA (L.) KURZ- AN IMPORTANT AYURVEDIC PLANT
Pankaj Kumar 1, 2, Kanwaljeet Singh 1, Zohra Batool 1, 2, Javaid Fayaz Lone 1, 2 and Sumeet Gairola 1, * 2
Plant Science Division 1, CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu - 180001, Jammu and Kashmir, India
Academy of Scientific and Innovative Research (AcSIR) 2, Ghaziabad - 201002, Uttar Pradesh, India.
ABSTRACT: Raw herbal samples used in the herbal medicine industry need to be properly identified for use in an herbal preparation. Plant parts of Woodfordia fruticosa (L.) Kurz belonging to the Lythraceae family, apart from some commercial uses, is known for its medicinal value in ethno medicines and various Indian traditional medicine systems, including Ayurveda. Flowers are reported high trade value (2000-5000 MT), are used in some Ayurvedic formulations such as Atisara, Raktapitta, Trsna, Vrana, Visarpa, Arjunarishta (Parthadyarishta), and Partharishtam. The present study aimed at botanical characterization and identification of raw leaf, flower, and stem bark herbal samples of Woodfordia fruticosa. Macroscopic and microscopic characters were studied using stereomicroscope and compound microscope. The morpho-anatomical description was provided for flower, leaf, and stem bark samples. Anatomical study of a leaf with a crescent-shaped vascular bundle, three different types of trichomes, and bark with linearly arranged rosette crystals crossing uniseriate medullary rays longitudinally were observed as characteristic features. Powder organoleptic and microscopic characters were described for each studied herbal sample. Characters compiled in the present study can be used as reference standards for future identification of raw leaf, flower, and stem bark samples of Woodfordia fruticosa.
| Keywords: |
Dried raw herbal sample, Identification problem, Macroscopic and Microscopic characterization, Reference standards.
INTRODUCTION: Woodfordia fruticosa (L.) Kurz belonging to the family Lythraceae, occurs in tropical and subtropical parts throughout India, especially in the Himalayas and Gangetic plains up to an altitude of 1500 m asl; and also cultivated in gardens 1, 2. It is commonly known as Fire flame bush, Dhavi, Dhaatkikephool, Shiranjitea, Thawi, and several other names 3.
Trade names of flower samples are Dhaiphool, Dhavadiphool, Dhataki; and are reported with a high annual trade value of 2000-5000 metric tonnes in Indian herbal market 4. Commercially, flowers known to yield a red dye used to color silks 3.
Leaves are reported to yield pink and red dye (due to the presence of Lawsone, 2-hydroxy naphthoquinone)5, milk enhancement in livestock 6 and also in perfume, leather, and textile industries 7. Different plant parts of W. fruticosa especially flowers, stem bark, and leaves are reported with medicinal importance. Flowers known used as astringent, antipyretic, appetizer, blood purifier, used in dysentery, diarrhea, leucorrhoea, skin problems, fever, asthma, liver disorder, rheumatism, menorrhagia, and inflammatory conditions 8-13. Flowers are known to have antibacterial 14, 15, antiviral (antiEV71) 16, hepato-protective 17, 18, immunomodulatory 19, antihyper-glycemic 20, antifertility 21, antitumor activity 22, cytotoxicity, anti-inflammatory, and analgesic properties 23. Flowers of W. fruticosa are known used in Ayurvedic formulations including Arjunarishta (Parthadyarishta) 24, in Ayurvedic drug ‘Partharishtam’, 25 and other Ayurvedic formulations including Atisara, Raktapitta, Trsna, Vrana, Visarpa and known with therapeutic uses of Atisara, Raktapitta, Trsna, Vrana, Visarpa 2. Flowers are the key ingredient used in the alcoholic preparation of “Asavas-Arishtas”26. Several species of yeast (such as Pichia anomola, Aspergillus niger, and Saccharomyces cerevisiae, etc. have been reported from W. fruticosa 27, 28. Stem bark was reported used in jaundice 29, diarrhoea 30. Leaves are used as disinfectant 31, 32, used in fever 15, 33, rheumatism 34, hemoptysis 35, ulcers 36, and in gall bladder problems 12. Leaves are known to have anti-microbial compounds 37, stem bark with analgesic activity 38; leaves and stem bark with antibacterial 39 and antidiabetic activity 40.
Various parts of W. fruticosa are reported to have tannins, such as in bark (20-27% tannins), flowers (24.1% tannins), and leaves (12-20% tannins) 41. Chemically, leaves are known to have flavonoids 42, essential oil 43, and phenolic compounds 44, 45. Leaves and flowers are known to have polyphenols 46, flowers have tannins, phytophenols, anthocyains 47 and Woodfordina ABC (tannins) 48, bark with C-glucoside and bergenin 9. In the herbal drug industry, proper identification and authentication of raw herbal samples are essential to ensure the quality, safety, and efficacy of herbal medicines 49-51. Several pharmacopeia monographs are known to use macro-morphological and organoleptic characters of herbal drugs in the correct identification of species 52. Botanical identification methods are considered as simple, easy, time, and cost-effective methods in the correct identification of raw herbal drugs 53-54.
The present study involved detailed qualitative and quantitative characterization of macroscopic and microscopic features of the leaf, stem bark, and flower samples. Botanical characters compiled in the present study can be used as reference standards for future identification of raw herbal samples of Woodfordia fruticosa used in herbal medicines preparations.
MATERIAL AND METHODS: Plant material was collected from two different locations of the U.T. of J&K's Table 1. Plant material was collected for herbarium sheet preparation, for raw crude herbal samples, and for botanical studies. Herbarium sheets were prepared following standard herbarium procedures 55. Duly identified herbarium sheets were submitted to internationally recognized Janaki Ammal Herbarium (RRLH) at the Indian Institute of Integrative Medicine (CSIR-IIIM), Jammu. Oven-dried raw herbal samples (of flowers, stem bark, and leaves) were submitted to the Crude Drug Repository at CSIR-IIIM Jammu. Herbarium and crude drug accession numbers have been provided in Table 1.
In botanical studies, macroscopic and microscopic characters of flower, stem bark, and leaves were studied using stereo-microscope (LEICA S9i) and compound microscope. For anatomical studies, transverse sections (T.S.) of leaf and stem bark samples were obtained by freehand sectioning using a razor blade. Obtained fine sections were stained according to Kumar et al., 56, with some modifications. Thin T.S. were dehydrated in different series of alcohol gradients (30%, 50%, and 70% alcohol, each for 10-15 min), stained in safranin (5-7 min), decolorized in 70% alcohol(5-10 min), staining in fast green (2-3 min) and then were again decolorized in 70% alcohol for 5-10 min. The sections were dehydrated in 90% alcohol followed by absolute alcohol (each for 5-7 min), mounted in Canada balsam, and observed under a compound microscope (Leica DM 750) with an associated camera (LEICA ICC50E). All micro-metric measurements were performed by LEICA LAS V 4.9.0 software. For powder study, samples were crushed to a powder, passed through a fine sieve, and studied in water-mounted slides under a compound microscope. An iodine test was performed to detect the presence of starch grains in powder samples. Organoleptic characters of leaf flower and stem bark samples were also noted.
RESULTS:
Botanical Description: The plant is bushy, spreading, semi-deciduous, perennial, under shrub or shrub, 1-3 m high, growing on rocky, dry areas in hilly areas Fig. 1A. Plants bear several bright red flowers in axillary clusters along the branches and twigs.
Morphological Characters:
Leaf and Flower: Leaves are ovate-lanceolate to ovate, opposite or sub opposite, 8-12 × 2.4-3.5 cm, whitish and tomentose abaxially, texture leathery, margin smooth, base subcordate, apex pointed and slightly curved, petiole nearly absent Fig. 1L. Flowers bright red colored, axillary, present in paniculate-cymose clusters (of 3-15 flowers) Fig. 1B, C, dried flowers dull red Fig. 1J, with short pedicels (0.4-0.6 cm) Fig. 1D. Flower buds with 6 angular protrusions, mature flower actinomorphic (appear slightly zygomorphic) with tubular corolla (16-20 mm long, 1.6-2.7 mm thick); sepals and petals six in number, sepals triangular-shaped, small tooth-like protrusions (0.9-1.2 mm), petals are narrowly linear slightly longer than the calyx-teeth (2.5-3.5 mm) Fig. 1F, G. Stamens are 12 in number, 0.8-1.4 cm, epipetalous, inserted little above ovary base Fig. 1E. Pistil size ranged from 1.6-1.9 cm, stigma bifid Fig. 1H, ovary bilocular, anthers versatile Fig. 1I. Anthers fall off easily from the filament in dried flower samples Fig. 1D, E, and 2C.
Stem Bark: The bark is thin (0.5 cm or more in thickness), smooth, reddish-brown colored, freshly peeled bark dark brown on the outer side and light creamy colored on the inner side. Bark surface observed with transverse and longitudinal cracks, peeling off in flakes (near the base of the stem) and in thin and fibrous strips (middle stem region) become curved on drying Fig. 1K. Dried bark samples are irregularly curved Fig. 1K with the outer surface dark brown colored and inner surface reddish-grey colored.
Microscopic Characters:
Flower: Cut view of flower showed superior and bilocular ovary (0.5-0.65 cm), with several rounded to oval ovules Fig. 2C, F, style elongated (0.9-1.2 cm), bifid stigma Fig. 1H, and with versatile anthers Fig. 1I.
Stem Bark: T.S. of bark showed outer flaky, thin cork zone (81.08 ± 6.45 µm) with compactly packed cells. Cork zone followed by a broad parenchymatous secondary cortex zone (166.66 ± 12.43 µm) with oval to slightly transversally elongated thick-walled cells. Cortex followed by a continuous thick phloem zone with phloem cells interspersed in cortex cells. Phloem formed a major part (thickness of 630.59 ± 17.06 µm) in studied cross-section of bark (of a total thickness of 788.64 ± 5.83 µm). Phloem comprised of oval-shaped cells interspersed with vertical medullary rays.
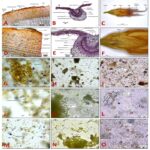
Phloem cells observed with several rosette crystals arranged in a transverse parallel row-like pattern. Medullary rays were nearly uniseriate on the inner phloem and showed dilatation growth (up to 4-10 cells wide) in the outer phloem near the cortical region. The inner zone of the phloem was comprised of the phloem fiber zone, followed by the xylem region consisting of well distinct xylem fibers and vessels Fig. 2A, D. Quantitative microscopic characters are shown in Table 2 and 3.
Transverse section of leaf blade (from midrib region) showed typical dicot leaf anatomy with central midrib region (with a notch in the center) and wing-like extended lamina region Fig. 2B. The Lamina region consisted of a single-layered, cuticularised upper epidermis with rectangular-shaped cells. Lamina epidermis consisted of few oval-shaped glandular trichomes and few curved, pointed trichomes with a broad base and abruptly tapering tip Fig. 2E. Epidermis followed by palisade layer with compactly packed elongated cells, then by spongy parenchyma zone. The lower epidermis region consisted of several uniformly thickened curved trichomes. The Midrib region consisted of a single-layered cuticularised epidermis followed by an inner collenchymatous patch and then by a broad parenchymatous tissue zone. The vascular zone was crescent-shaped with well distinguishable xylem facing the upper epidermis, followed by a less distinct phloem zone sheathed by a well-differentiated continuous sclerenchymatous zone. Xylem vessels were present in linear rows with comparatively broader vessel lumen diameter towards the abaxial side than the adaxial side. The vascular zone was followed by a broad parenchymatous zone (8-10 cell wide) with rosette crystals in some cells, aninner 2-3 cell wide collenchymatous tissue zone, and a single-layered lower epidermis. Quantitative microscopic characters are shown in Table 4.
FIG. 1: MORPHOLOGICAL STUDIES ON RAW HERBAL SAMPLES OF W. FRUTICOSA, A). PLANT HABIT, B). FLOWERS CLUSTER ON THE PLANT, C). FRESH RAW FLOWER SAMPLES, D). SINGLE FLOWER MORPHOLOGY, E). THE FLOWER OPENED (SHOWING STAMEN ATTACHMENT TO FLOWER TUBE), F). FLOWER PART SHOWING SEPALS, PETALS, AND STAMEN FILAMENTS, G). SEPAL, PETAL MORPHOLOGY AND FLOWER TUBE, H). STIGMA MORPHOLOGY, I). ANTHER MORPHOLOGY, J). DRIED FLOWER SAMPLES, K). DRIED STEM BARK SAMPLES, L). DRIED LEAF SAMPLES, M). FLOWER POWDER SAMPLE, N). STEM BARK POWDER SAMPLE, O). LEAF POWDER SAMPLE
Powder Study: Organoleptic features including color, odor, texture, and taste of each drug sample (flower, stem bark, and leaf samples) were observed characteristic. Organoleptic characters of flower leaf and stem bark samples are provided in Table 1. A microscopic study of powder samples was observed with characteristic features for each drug type. Microscopic powder study of leaf samples was observed with cork cell fragments, non-glandular trichomes, few golden yellow fragments, and few rosette crystals; flower samples with few cork cells, several rounded pollen grains (mean size of 17.64 ± 0.21 × 16.88 ± 0.27 µm), a few unicellular trichomes, few cork cells, and few prismatic crystals. Microscopic powder study of stem bark sample was observed with cork cell fragments, starch grains (mean size of 12.18 ± 0.74 × 9.71 ± 0.47 µm), and rosette crystals (mean size of 14.58 ± 0.96 × 12.46 ± 0.84 µm). Iodine test revealed abundant starch grains in stem bark powder sample while starch was not detected in leaf and flower powder sample. The mean size and range of starch grains, rosette crystals, and pollen grains in studied powder samples are shown in Table 3 and 4.
FIG. 2: MICROSCOPIC STUDIES ON RAW HERBAL SAMPLES OF W. FRUTICOSA, A). PLANT HABIT, B). FLOWERS CLUSTER ON THE PLANT, C). FRESH RAW FLOWER SAMPLES, D). SINGLE FLOWER MORPHOLOGY, E). THE FLOWER OPENED (SHOWING STAMEN ATTACHMENT TO FLOWER TUBE), F). FLOWER PART SHOWING SEPALS, PETALS, AND STAMEN FILAMENTS, G). SEPAL, PETAL MORPHOLOGY AND FLOWER TUBE, H). STIGMA MORPHOLOGY, I). ANTHER MORPHOLOGY, J). DRIED FLOWER SAMPLES, K). DRIED STEM BARK SAMPLES, L). DRIED LEAF SAMPLES, M). FLOWER POWDER SAMPLE, N). STEM BARK POWDER SAMPLE, O). LEAF POWDER SAMPLE
TABLE 1: COLLECTION DETAILS AND POWDER STUDIES OF DIFFERENT SAMPLES OF WOODFORDIA FRUTICOSA
| Collection details of plant samples | |||
| GPS location | Herbarium accession number | CDR accession number | |
| Nandini WLS (J&K) | 32°50.674N, 074°56.660E (529m asl) | 23811 | Flower (4174), Stem (4175 bark), Leaves (4220) |
| Pallan (Billawar, J&K) | 32°33.320N, 75°33.751E (633m asl) | 23395 | |
| Powder organoleptic characters | |||
| Flower | Stem bark | Leaf | |
| Colour | Soil like brown colored
(Figure 1M) |
Soil colored (Figure 1N) | Light green to creamish green (Figure 1O) |
| Odor | Slightly characteristic odor | No characteristic odor | Characteristic odor |
| texture | Slight granular | Sand like granular | Smooth to slightly rough |
| Taste | No characteristic taste | Slightly bitter with a rough mouthfeel | Characteristic, slightly bitter |
TABLE 2: QUANTITATIVE MICROSCOPIC CHARACTERS OF THE T.S. OF STEM BARK OF WOODFORDIA FRUTICOSA
| Character | Min | Max | Mean (±S.D.) |
| Stem bark (µm) | |||
| T.S. thickness | 746.49 | 818.49 | 788.64±5.83 |
| Cork thickness | 55.36 | 115.62 | 81.08±6.45 |
| Cortex thickness | 123.66 | 250.96 | 166.66±12.43 |
| Phloem thickness | 514.12 | 683.96 | 630.59±17.06 |
| Intermedullary ray width | 19.93 | 80.08 | 48.81±6.34 |
TABLE 3: QUANTITATIVE MICROSCOPIC CHARACTERS OF STEM BARK OF WOODFORDIA FRUTICOSA
| Min | Max | Mean (±S.D.) | Min | Max | Mean (±S.D.) | |
| Stem bark cell size (µm) | Length | Breadth | ||||
| Cork | 11.69 | 21.45 | 17.50±1.06 | 6.76 | 16.80 | 12.16±0.89 |
| Cortex | 21.07 | 43.13 | 30.08±2.07 | 9.03 | 16.36 | 13.20±0.69 |
| Phloem parenchyma | 12.33 | 22.47 | 16.07±1.00 | 10.14 | 13.65 | 11.99±0.43 |
| Stem bark medullary ray | 474.01 | 709.03 | 626.25±30.25 | 13.30 | 24.19 | 17.48±1.08 |
| Starch grains | 9.69 | 16.65 | 12.18±0.74 | 7.91 | 11.91 | 9.71±0.47 |
| Rosette crystals | 9.97 | 19.13 | 14.58±0.96 | 7.29 | 15.24 | 12.46±0.84 |
TABLE 4: QUANTITATIVE MICROSCOPIC CHARACTERS OF FLOWER AND LEAF OF WOODFORDIA FRUTICOSA
| Min | Max | Mean (±S.D.) | Min | Max | Mean (±S.D.) | |
| Flower characters | ||||||
| Pollen grains | Equatorial axis | Polar axis | ||||
| 16.56 | 18.57 | 17.64±0.21 | 15.63 | 18.07 | 16.88±0.27 | |
| Leaf characters | ||||||
| Length | Breadth | |||||
| Upper epidermis (midrib region) | 5.85 | 9.09 | 7.17±0.32 | 3.24 | 6.42 | 5.08±0.39 |
| Upper epidermis (lamina region) | 14.22 | 23.85 | 18.66±1.05 | 12.47 | 24.5 | 17.37±1.36 |
| Lower epidermis (midrib region) | 5.29 | 9.87 | 7.12±0.45 | 4.86 | 7.93 | 6.77±0.30 |
| Adaxial cortical cell size | 11.69 | 24.1 | 18.48±1.16 | 8.36 | 17.31 | 13.10±0.91 |
| Abaxial cortical cell size | 16.26 | 45.36 | 26.97±3.21 | 12.34 | 34.20 | 19.99±2.81 |
| Trichome (Curved) | 25.10 | 94.24 | 50.32±6.88 | 8.86 | 18.65 | 11.66±1.13 |
| Trichome (Straight) | 42.42 | 181.72 | 79.94±16.22 | 17.93 | 43.88 | 29.65±2.22 |
| Palisade thickness | 59.10 | 73.45 | 65.28±1.60 | |||
| Xylem length | 65.75 | 114.09 | 93.11±5.13 | |||
| Xylem vessel diameter | 10.24 | 29.56 | 19.20±1.90 | |||
DISCUSSION: Identification of entirely unknown raw herbal samples without a reference standard is considered problematic 57. Detailed macroscopic and microscopic characterization, including qualitative and quantitative features, can be more useful in the identification of raw herbal samples 54, 58. Macroscopic and microscopic characterization has been performed in different types of herbal samples such as whole plant 59, heartwood 60; leaves 61, root 62, rhizome 63, stem bark 64, flowers 26, etc. Botanical-based identification methods vary for different plant samples 65. Anatomical characters have been used for the identification of raw leaf and bark drug samples in several species 66-68. Kotina et. al., 68, observed characters such as trichomes, sclereids, secretory canals, druse crystals, brown contents in parenchyma cells as diagnostic microscopic features in the identification and differentiation of raw leaf and bark herbal material from adulterant samples.
For identification of stem bark, macroscopic characters (such as shape, size, surface color, texture, etc.), microscopic features (of rhytidome, cork, cortex, ray dilation, sclereids in phelloderm, secondary phloem, phloem fibers, starch grains, the shape of crystals, stone cells, tannins, etc.) and powder features were known helpful in species characterization 69-72.
In the present study, botanical identification studies with macroscopic, microscopic, and powder characterization were performed on the leaf, stem bark, and flower samples. Studies performed included descriptions of qualitative and quantitative macroscopic and microscopic botanical characters. Botanical studies with anatomical characterization have been done in some previous studies on leaf samples 73 and flower samples 26, 74, 75. Leaf anatomical characters observed in the present study also corresponded with anatomical features studied by Birajdar et al., 73 In the microscopic study of flower powder of W. fruticosa, Baravalia et al., 75, observed unicellular trichomes, rosette, and calcium oxalate crystals. However, in the present study, rosette crystals were not observed in flower powder microscopic study. Microscopic studies for stem bark samples were described for the first time in the present study. In the present study, the anatomical study of leaf samples revealed some characteristic features, including a notch in the central region of the midrib, crescent-shaped vascular bundle, varied types of trichomes (oval-shaped glandular trichomes; curved and straight non-glandular trichomes). Transverse section of stem bark was observed with uniseriate longitudinal medullary rays (with dilation growth near cortical region) and phloem parenchyma cells with rosette crystals in a transverse arrangement.
Powder study of stem bark was observed with few cork cell fragments, abundant oval to elongated starch grains, and rosette crystals. Starch grains were not detected in leaf and flower powder samples.
CONCLUSION: The present study involved detailed morphological, anatomical, and powder studies with qualitative and quantitative characterization for the raw leaf, flower, and stem bark samples of W. fruticosa. Some characteristic features of the leaf (crescent-shaped vascular bundles with rosette crystals in cortex cells), flower (macroscopic, microscopic features), and stem bark samples (characteristic arrangement of rosette crystals in phloem cells to uniseriate medullary rays) have been summarised in the present study. Botanical characters described in the present study can be used as a rapid reference identification standard for future identification of raw samples of W. fruticosa in fresh as well as dried form.
ACKNOWLEDGEMENT: The authors thank Director IIIM Jammu for providing the necessary facilities to carry out the work. The authors are thankful to the Council of Scientific and Industrial Research (CSIR), Government of India, for financial assistance under the Phytopharmaceutical Mission (HCP-0010). PK acknowledges the financial support provided by CSIR in the form of JRF/SRF fellowships.
CONFLICTS OF INTEREST: No
REFERENCES:
- Kirtikar KR and Basu BD: Indian Medicinal Plants. Part 1-3, L.M. Basu, Allahabad, India. 1935.
- API: The Ayurvedic Pharmacopoeia of India. Part-I, Vols, I to V. Government of India. Ministry of Health and Family Welfare, Department of AYUSH, India. 2001.
- Kumar D, Sharma M, Sorout A, Saroha K and Verma S: Woodfordia fruticosa : a review on its botany, chemistry and biological activities. Journal of Pharma-cognosy and Phytochemistry 2016; 5(3): 293-98.
- NMPB: Traded Medicinal Plant Database. http://envis. frlht.org/traded-medicinal-plants-database.php (accessed 28th July 2020). 2020.
- Singh R and Srivastava S: A critical review on extraction of natural dyes from leaves. International Journal of Home Science 2017; 3(2): 100–103.
- Salave AP and Reddy PG: Documentation of traditional knowledge on fodder uses by the native Inhabitants in Beed District (M.S.) India. Life Sci Leafl 2012; 9: 24–34.
- Gaur RD: Traditional dye yielding plants of Uttarakhand, India. Natural Product Radiance 2008; 7: 154–65.
- Finose A and Devaki K: Phytochemical and Chromato-graphic studies in the flowers of Woodfordia fruticosa (L) Kurz. Asian J of Plant Sci and Research 2011; 1(3): 81-85.
- Khare CP: Indian medicinal plants: An illustrated dictionary, Springer 2007.
- Tambekar DH and Khante BS: Antibacterial properties of traditionally used medicinal plants for enteric infections by Adivasi’s (bhumka) in Melghat forest (Amravati district). International Journal of Pharmaceutical Sciences and Research 2010; 1(9): 120-28.
- Bhushan B and Kumar M: Ethnobotanically Important Medicinal Plantsof Tehsil Billawar, District Kathua, J&K, India. J of Pharmac and Phytochemistry 2013; 2(4): 14-21.
- Gairola S, Sharma J and Bedi YS: A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J of Ethnopharmacology 2014; 155: 925-86.
- Bhatia H, Sharma YP, Manhas RK and Kumar K: Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J of Ethnophar 2014; 151: 1005-8.
- Parekh J and Chanda S: In-vitro antibacterial activity of the crude methanol extract of Woodfordia fruticosa kurz flower (Lytheraceae). Brazalian Journal of Microbiology 2007; 38: 204-07.
- Kumaraswamy MV, Kavitha HU and Satish S: Antibacterial Potential of Extracts of Woodferdia fruticosa Kurz on Human Pathogens. World Journal of Medical Sciences 2008; 3(2): 93-96.
- Choi HJ, Song JH, Park KS and Baek SH: In-vitro anti-enterovirus 71 activity of gallic acid from Woodfordia fruticosa flowers. Lett in App Microbiol 2010; 50: 438-40.
- Chandan BK, Saxena AK, Shukla S, Sharma N, Gupta DK and Singh K: Hepatoprotective activity of Woodfordia fruticosa kurz flowers against Carbon tetrachloride induced hepatotoxicity. Journal of Ethnopharmacology 2008; 119: 218-24.
- Baravalia Y, Chanda S and Kaneria M: Hepatoprotective effect of Woodfordia fruticosa Kurz flowers. Asian Pacific Journal of Tropical Medicine 2011; 4: 673-79.
- Shah AS and Javekar AR: In-vitro and In-vivo immunostimulatory activity of Woodfordia fruticosa flowers on non specific immunity. Pharmaceutical Biology 2010; 48: 1053-58.
- Verma N, Amresh G, Sahu PK, Rao V and Singh AP: Antihyperglycemic activity of Woodfordia fruticosa Kurz flowers extracts in glucose metabolism and lipid peroxidation in streptozotocin induced diabetic rats. Indian Journal of Experimental Biology 2012; 50: 351-58.
- Kushlani H, Tatke P and Singh KK: Antifertility activity of dried flowers of Woodfordia fruticosa Indian Journal of Pharmaceutical Sciences 2006; 68: 512-29.
- Yoshida T, Chou T, Nitta A, Miyamoto K, Koshiura R and Okuda T: Woodfordin C, a macro-ring hydrolysable tannin dimer with antitumor activity, and accompanying dimmers from Woodfordia fruticosa Chemical and Pharmaceutical Bulletin 1990; 38(5): 1211-17.
- Baravalia Y, Kumar YV and Chanda S: Brine shrimp cytotoxicity, anti inflammatory and analgesic properties of Woodfordia fruticosa kurz flowers. Iranian Journal of Pharmaceutical Research 2012; 11: 854-61.
- Singh H, Mishra SK and Pande M: Standardization of Arjunarishta formulation by TLC method. International J of Pharma Sci Review and Research 2010; 2(1): 25-28.
- Sadhanandham S, Narayanan G, Rao MRK, Prabhu K, Jones S, Ravi A and Dinakar S: GC-MS Analysis and Antioxidant studies of an Ayurvedic drug, Partharishtam. International Journal of Pharmaceutical Sciences Review and Research 2015; 34(2): 273-81.
- Admani M, Kumar KNS and Mallya SV: Pharmacognostic characterisation of flowers Woodfordia fruiticosa (Dhataki Pushpa) used as fermentation initiators. Journal of Ayurvedic and Herbal Medicine 2015; 1(1): 09–12.
- Vohra A and Satyanarayana T: A cost-effective cane molasses medium for enhanced cell-bound Phytase production by Pichia anomala. Journal of Applied Microbiology 2004; 97: 471-76.
- Manwar J, Mahadik K, Paradkar A, Sathiyanarayanan L, Vohra M and Patil S: Isolation, biochemical and genetic characterizations of alcohol-producing yeasts from the flowers of Woodfordia fruticosa. Journal of Young Pharmacists 2013; 5(4): 191-94.
- Sharma J, Gairola S, Gaur RD and Painuli RM: The treatment of jaundice with medicinal plants in indigenous communities of the Sub-Himalayan region of Uttarakhand, India. Journal of Ethnopharmacology 2012; 143: 262-91.
- Das PK, Goswami S, Chinniah A, Panda N, Banerjee S, Sahu NP and Achari B:Woodfordia fruticosa: Traditional uses and recent findings. J of Ethno 2007; 110: 189-99.
- Khan AM, Qureshi RA, Gillani SA and Ullah F: Antimicrobial activity of selected medicinal plants of Margalla Hills, Islamabad, Pakistan. Journal of Medicinal Plants Research 2011; 5: 4665-70.
- Xavier F, Arun VR and Rose F: Ethnopharmacological studies on the medicinal plants used by tribal inhabitants of Meenagadi region in Wayanadu district of Kerala, South India. International Journal of Medicinal Plants Research 2012; 1: 58-62.
- Kaur R and Kaur H: The Antimicrobial activity of essential oil and plant extracts of Woodfordia fruticosa. Archives of Applied Sciences Research 2010; 2: 302-9.
- Jeyaprakash K, Ayyanar M, Geetha KN and Sekar T: Traditional uses of medicinal plants among the tribal people in Theni District (Western Ghats), Southern India. Asian Pacific J of Tropical Biomedicine 2011; 1: 20-5.
- Dubey D and Padhy RN: Surveillance of multidrug resistance of two gram-positive pathogenic bacteria in a teaching hospital and in-vitro efficacy of 30 ethnomedicinal plants used by an aborigine of India. Asian Pacific Journal of Tropical Disease 2012; 2: 273-81.
- Bharati KA and Sharma BL: Some Ethnoveterinary plant records for Sikkim Himalaya. Indian Journal of Traditional Knowledge 2010; 9: 344–6.
- Dubey D, Patnaik R, Ghosh G and Padhy RN: In-vitro antibacterial activity, GC-MS analysis of Woodfordia fruticosa leaf extract and host toxicity testing with in-vitro cultured lymphocytes from human umbilical cord blood. Osong Public Health and Research Perspectives 2014; 5(5): 298-12.
- Rose BN and Prasad NK: Analgesic activity of extracts of Woodfordia fruticosa stems bark in animal models. Indian Journal of Biological and Pharma Res 2013; 4: 175-80.
- Chougale AD, Padul MV, Arfeen S and KakadSl: Antibacterial activity directed fractionation of Woodfordia fruticosa leaves. J Medi Plants 2009; 8(31): 75-81.
- Beck NR and Namdeo KP: Anti diabetic activity of aqueous extracts of leaves and stem barks of Woodfordia fruticosa in animal model. World Journal of Pharmaceutical Sciences 2015; 3(3): 468-74.
- Rastogi RP and Mehrotra BN: Compendium of Indian Medicinal Plants. Vol-1, Central Drug Research Institute, Lucknow 1999.
- Khan AM, Qureshi RA, Ullah F, Khan ZS and Khan J: Flavonoids distribution in selected medicinal plants of Margalla Hills and surroundings. Pakistan Journal of Botany 2012; 44: 1241–5.
- Hemraj, Gupta A, Thakur A and Upmanyu N: Hydro distillation of Stephania glabra tubers and Woodfordia fruticosa Asian Journal of Pharmaceutical and Clinical Research 2012; 5: 105–7.
- Bhatt LR, Lim JA, Lim CH and Baek SH: Antimicrobial and antiradical activity of Nepalese medicinal plants. Korean J of Oriental Physio and Patho 2007; 21: 1564-8.
- Bajracharya AM, Yami KD, Prasai T, Basnyat SR and Lekhak B: Screening of some medicinal plants used in Nepalese traditional medicine against enteric bacteria. Sci World 2008; 6: 107-10.
- Nair AGR, Kotiyal JP, Ramesh P and Subramanian SS: Polyphenols of the flowers and leaves of Woodfordia fruticosa. Indian Journal of Pharmacy 1976; 38: 110-11.
- Yoshida T, Chou T, Nitta A and Okuda T: Tannins and related polyphenols of lythraceous plants III hydrolysable tannins oligomers with macro cyclic structures and accompanying tannins from Woodfordia fruticosa Chemical and Pharmaceutical Bulletin 1992; 40: 2023-30.
- Yoshida T, Chou T, Nitta A and Okuda T: Woodfordina ABC. dimeric hydrolysable tannins from Woodfordia fruticosa Heterocycles1989; 29: 2267-71.
- Zhao ZZ, Hu YN, Liang ZT, YuenJPS, Jiang ZH and Leung KSY: Authentication is fundamental for standardization of Chinese medicines. Planta Medica 2006; 72: 865-74.
- Shinde VM, Dhalwal K, Potdar M and Mahadik KR: Application of quality control principles to herbal drugs. International Journal of phytomedicine 2009; 4–8.
- Sahoo N, Manchikanti P and Dey S: Herbal drugs: standards and regulation. Fitoterapia 2010; 81(6): 462-71.
- Upton R, David B, Gafner S and Glasl S: Botanical ingredient identification and quality assessment: strengths and limitations of analytical techniques. Phytochemistry Review 2019; 19: 1157-77.
- Li J, Yi T, Lai HS, Xue D, Jiang H, Peng HC and Zhang H: Application of microscopy inauthentication of traditional Tibetan medicinal plant Halenia elliptica. Microscopy Research Technique 2008; 71(1): 11-19.
- Akbar S, Hanif U, Ali J and Ishtiaq S: Pharmacognostic studies of stem, roots and leaves of Malva parviflora Asian Pacific J of Tropical Biomedi 2014; 4(5): 410-15.
- Rao RR and Sharma BD: A manual for herbarium collections. Botanical Survey of India 1990.
- Kumar P, Singh K and Gairola S: Botanical standardization of raw herbal drug Pashanabheda [Bergenia ciliata (Haw.) Sternb.] used in Indian Systems of Medicine. Plant Archives 2020; 20(2): 8645-52.
- Ghorbani A, Saeedi Y and Boer HJ: Unidentifiable by morphology: DNA barcoding of plant material in local markets in Iran. PLoS ONE 2017; 12.
- Hassan LM, Galal TM, Farahat EA and El-Midany MM: The biology of Calotropis procera (Aiton) W.T., Trees 2015; 29: 311-20.
- Wang Y, Wen Y and Gao J: Anatomy and microscopic characteristics of Picris japonica. Revista Brasileira de Farmacognosia 2018; 28: 640-46.
- Sundharamoorthy S, Govindarajan N, Chinnapillai A and Raju I: Macro-Microscopic Atlas on Heartwood of Santalum album (Sandalwood). Pharmacognosy Journal 2018; 10(4): 730-33.
- Kumar KNS: Macro-microscopic examination of leaves of Cinnamomum malabatrum (Burm. f.) Blume sold as Tamalapatra. AYU 2013; 34(2): 193-99.
- Manohan R, Palanuvej C and Ruangrungsi N: Pharmacognostic specifications of five root species in Ben-Cha-Moon-Yai remedy: Thai traditional medicine remedy. Pharmacognosy Journal 2013; 5: 46-55.
- Kala C, Ali SS and Chaudhary S: Comparative pharmacognostical evaluation of Costus speciosus (wild ginger) and Zingiber officinale (ginger) rhizome. International Journal of Current Pharmaceutical Research 2016; 8(4): 19-23.
- Sharma N, Singh S and Singh SK: Pharmacognostical standardization and preliminary phytochemical investigations on Acacia auriculiformis Cunn. Ex. Benth stem bark. J of Medi Plants Stud 2017; 5(1): 398-02.
- Evans WC: Trease and Evans pharmacognosy. 16th edition, 2009; 541-50.
- Coelho VPM, Leite JPV, Nunes LG and Ventrella MC: Anatomy, histochemistry and phytochemical profile of leafand stem bark of Bathysa cuspidate (Rubiaceae). Australian Journal of Botany 2012; 60: 49-60.
- Barkatullah, Ibrar M, Jelani G and Ahmad I: Leaf, stem bark and fruit anatomy of Zanthoxylum armatum (Rutaceae). Pak Journal of Botany 2014; 46(4): 1343–49.
- Kotina EL, Van Wyk BE, Tilney PM, Anatomy of the leaf and bark of Warburgiasalutaris (Canellaceae), animportant medicinal plant from South Africa. South African Journal of Botany 2014; 94: 177-81.
- Eltahir AS andAbuReish BI:Comparative morphological and anatomical studies of the barks of three Albizzia species. Journal of Chemical and Pharmaceutical Research 2010; 2(3): 260-68.
- Sreedhar S, Kumar UP and Shree ABR: Pharmacognostic Analysis of Stem Bark of Combretum albidum Don; An Unexplored Medicinal Plant. Pharmacognosy Journal 2012; 4(28): 13-18.
- Mota GS, Sartori CJ, Miranda I, Quilho T, Mori FA and Pereira H: Bark anatomy, chemical composition and ethanol-water extract composition of Anadenanthera peregrina and Anadenanthera colubrina. Plos One 2017; 12(12): 1–14.
- Somavilla NS, Fagg CW and Brandao MGL: Morpho-anatomy of native species used as substitute of quina (Cinchona spp.) in Brazilian traditional medicine: Esenbeckia febrifuga. RevistaBrasileira de Farmacognosia 2018; 28: 223-27.
- Birajdar VV, Mhase AG, Gurav AM and Murthy SN: Preliminary pharmacognostic and phytochemical standardization of Dhataki [Woodfordia fruticosa (L.) Kurz.] leaves. Ayu 2014; 35(3): 309-15.
- Shome U, Mehrotra S and Sharma HP: Pharmacognostic studies on the flower of Woodfordia fruticosa Proceedings of the Indian Academy of Sciences (Plant Sciences) 1981; 90(4): 335-51.
- Baravalia Y, Nagani K and Chanda S: Evaluation of pharmacognostic and physicochemical parameters of Woodfordia fruticosa flowers. Pharmacognosy Journal 2011; 2(18): 13–18.
How to cite this article:
Kumar P, Singh K, Batool Z, Lone JF and Gairola S: Botanical studies on raw herbal samples of Woodfordia fruticosa (L.) kurz- an important ayurvedic plant. Int J Pharmacognosy 2020; 8(3): 129-37. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.8(3).129-37.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
6
129-137
821
1123
English
IJP
P. Kumar, K. Singh, Z. Batool, J. F. Lone and S. Gairola *
Plant Science Division, CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu, Jammu and Kashmir, India.
sumeetgairola@iiim.res.in
03 March 2021
28 March 2021
30 March 2021
10.13040/IJPSR.0975-8232.IJP.8(3).129-37
31 March 2021