BERGENIN – AN ACTIVE CONSTITUENT OF RIVEA ORNATA ROXB. AND ITS ANTIOXIDANT PROPERTY
HTML Full TextBERGENIN - AN ACTIVE CONSTITUENT OF RIVEA ORNATA ROXB. AND ITS ANTIOXIDANT PROPERTY
Vaishali J. Sharma * 1 and Piyush M. Patel 2
Department of Pharmacognosy 1, B. Pharmacy College Rampura Kakanpur, Godhra, Panchmahal - 389713, Gujarat, India.
Kalol Institute of Pharmacy 2, Kalol - 382721, Gujarat, India.
ABSTRACT: A simple TLC method has been developed for the simultaneous quantification of bergenin using HPTLC plate precoated with silica gel 60 F254. The method was developed in toluene: ethyl acetate: acetone (2:4:4 v/v) toluene: ethyl acetate: formic acid (4:6:1, v/v) and validated in terms of precision, repeatability, and accuracy. The isolated compounds were characterized using spectroanalytical techniques and found to be bergenin. The in-vitro antioxidant activity of the isolated compound was determined. For the antioxidant potential, two standard analytical protocols, namely, DPPH radical scavenging activity (RSA) and ferric reducing antioxidant power were adopted. The results showed that the compound was found to be a more potent antioxidant.
| Keywords: |
Rivea ornata Roxb., Phytochemical study, Antioxidant activity
INTRODUCTION: Medicinal plants can be cultivated within a home or community garden, and many grow wildly 1, 2. Some vegetables and fruits like berries, grapes, walnuts, olives, and foods like chocolate, wine, coffee and tea, and popcorn, and some breakfast cereals contain large amounts of healthful antioxidant substances called polyphenols. Bergenin is often included in thermogenic fat burners along with ingredients that stimulate norepinephrine release for its ability to enhance the breakdown of fat by this hormone. Bergenin may also be purchased by itself as an extract of Bergenia root 3, 4, 5, 6, 7, 8. Bergenin containing extracts have long been used as a folk medicine in several parts of Asia.
The molecular formula and the chemical structure of bergenin were confirmed by several spectroscopic methods and also by its synthesis 9. Bergenin exhibits antihepatotoxic, antiulcerogenic, anti-HIV, anti-arrhythmic, neuroprotective, anti-inflammatory and immunomodulatory properties 9.
Hyper physiological burden of free radical causes imbalance in homeostatic phenomenon between oxidants and antioxidants in the body. The imbalance leads to oxidative stress that is being suggested as the root cause of aging and various human diseases like arteriosclerosis, stroke, diabetes, cancer and neurodegenerative diseases such as Alzheimer’s and Parkinsonism. Therefore, research in recent past has accumulated enormous evidence advocating enrichment of body system with antioxidants to correct vitiated homeostasis and prevent onset as well as treat the disease caused due to free radical and related oxidative stress. Stress, smoking, drugs & diet generates excessive free radicals in the human body. Antioxidants have defined as the substance those in small quantities, able to prevent or greatly retard oxidation of easily oxidizable material such as fats. Antioxidants may exert their effect by different mechanisms such as suppressing the formation of active species by reducing hyperoxides (ROO) and H2O2 and also by sequestering metal ions scavenging active free radicals, repair and clearing damage.
MATERIALS AND METHODS:
Authentication and Collection of Plant: Dry plants of Rivea ornata were collected from the botanical garden of S. V. University, Tirupati in May 2011 and its authentication was confirmed by Botanist, Dr. Madhava Chetty, S. V. University Tirupati. Herbarium of the plant has been deposited at Department of Pharmacognosy, B. Pharmacy College, Rampura, Kakanpura, Dist. Panchmahal, Gujarat, India for future reference.
Preparation of Samples: Aerial parts of the plant were used for pharmacognostical studies. Aerial parts were collected, dried and powdered to 60# separately and stored in airtight containers and used for phytochemical and pharmacological studies.
Proximate Analysis: 10 - 15 Proximate analysis aids to set up a certain standard for dried crude drugs to avoid batch-to-batch variation and also to judge their quality. Their studies also give an idea regarding the nature of phytoconstituents present Table 2. Proximate analysis of these crude drug powders was carried out using methods prescribed in the Ayurvedic Pharmacopoeia of India by subjecting them to various determinations like:
- Total Ash
- Acid-insoluble ash
- Water soluble ash
- Alcohol soluble extractive value
- Water-soluble extractive value
- Loss of moisture content
Determination of Ash Value: Ash values of powder of aerial part of Rivea ornata Roxb. were determined by the following method:
a) Determination of Total Ash: 2 g of accurately weighed powder was incinerated in a crucible at a temperature 500 - 600 °C in a muffle furnace till carbon-free ash was obtained. It was then cooled, weighed and the percentage of ash was calculated with reference to the air-dried drug.
b) Determination of Acid Insoluble Ash: The total ash obtained above was boiled for 5min with 25 ml of 2M hydrochloric acid and filtered using an ashless filter paper to collect insoluble matter. The ash obtained was washed with hot water, and the filter paper was burnt to a constant weight in a muffle furnace. The percentage of acid-insoluble ash was calculated concerning the air-dried powered drug (60#).
c) Determination of Water-Soluble Ash: The total ash was boiled for 5min with 25 ml of water and insoluble matter collected on an ash-less filter paper washed with hot water and ignited for 15 min at a temperature not exceeded 450 °C in a muffle furnace. The difference in weight of ash and weight of water-insoluble matter gave the weight of water-soluble ash. The percentage of water-soluble ash was calculated concerning the air-dried powered drug.
Determination of Extractive Values: The following methods determined extractive values of powder of aerial parts of Rivea ornata:
d) Determination of Alcohol Soluble Extractive Value: 4 g of the air-dried powdered material were macerated with 100 ml of alcohol in a closed flask for 24 h, frequently shaking at an interval of 6 h. It was then allowed to stand for 18 h and filtered rapidly to prevent any loss during evaporation. 25 ml of the filtrate was evaporated to dryness in a porcelain dish and dried at 105 °C to a constant weight. The percentage of alcohol-soluble extractive was calculated concerning the air-dried drug.
e) Determination of Water-Soluble Extractive Value: 4 g of the air-dried powdered material was soaked in 100 ml of water in a closed flask for 1h with frequently shaking. It was then boiled gently for 1 h on a water bath; cooled and weighed and readjusted the weight. 25 ml of the filtrate was evaporated to dryness in a porcelain dish and dried at 105 °C to a constant weight. The percentage of water-soluble extractive was calculated concerning the air-dried powered drug (60#).
f) Determination of Moisture Content: Placed about 100 gm aerial part of Rivea ornata after accurately weighing in a tared evaporating dish. After placing the above-said amount of the drug in the tarred evaporating dish, dried at 105 °C for 5 h, and weighed. Continued the drying and weighing at one-hour interval until the difference between two successive weighing corresponds to not more than 0.25%. Constant weight was reached when two consecutive weighing after drying for 50mins.and cooling for 30 min in a desiccator, showed not more than 0.01 g difference.
Phytochemical Studies:
Preliminary Photo Profiles: 16, 17 Successive solvent extraction: 10g of the air-dried powdered plant material was successively extracted with the following solvents of increasing polarity in a Soxhlet apparatus.
- Petroleum ether (60 - 80 ºC)
- Ethyl acetate
- Chloroform
- Methanol
- Water
All the extracts were concentrated by distilling the solvents, and the extracts were dried in an oven at 50 °C. Each time before extracting with the next solvent, the marc was dried in an air oven below at 50 °C. The marc was finally macerated with water for 24 h to obtain the aqueous extract. The completion of the extraction was confirmed by evaporating a few drops of extract from the thimble on watch glass to observe that no residue remained after evaporation of the solvent. The liquid extracts obtained with different solvents were collected. The consistency, odor, color, appearance of the extracts and their percentage yield were noted. The extracts were then subjected to a various qualitative test using reported methods, to determine the presence of various phytoconstituents such as alkaloids, glycosides, flavonoids, carbohydrates, amino acids, saponins, sterols and terpenoids, cardiac glycosides, coumarins, carotenoids, tannins, phenolic compounds, fixed oils, and fats, etc. Table 4.
Qualitative Chemical Identification of Rivea ornata: 16 - 20 The extracts were subjected to various qualitative chemical tests to determine the presence of various phytoconstituents like alkaloids, glycosides, carbohydrates, phenolic and tannins, phytosterols, fixed oils and fats, proteins amino acids, flavonoids, saponins, etc. using reported methods.
Evaluation of Antioxidant Activity of Rivea ornata Roxb.:
Instruments: UV spectrophotometer (Shimadzu-UV-1601), Centrifuge Machine (Eltek-research centrifuge-TC-4100D).
Chemicals: All chemicals used for the study are purchased from SD-fine chemicals; India and all other reagent used were of analytical grade.
DPPH Radical Scavenging Activity: 21-23 Product extract and standard ascorbic acid solution (0.1 ml) of different concentrations viz. 10, 20, 40, 60, 80, 100 μg/ml was added to 3 ml of a 0.004% methanol solution of DPPH. An equal amount of methanol and DPPH served as control.
After 30 min incubation in the dark, absorbance was recorded at 517 nm, and the percentage inhibition activity was calculated from [(A0-A1)/A0] × 100, where A0 is the absorbance of the control, and A1 is the absorbance of the extract/standard. The antioxidant activity of the extract was expressed as IC50. The IC50 value was defined as the concentration (in μg/ml) of extracts that inhibits the formation of DPPH radicals by 50%. All the tests were performed in triplicate, and the graph was plotted with an average of three observations Table 5 and Fig. 5.
Ferric Reducing Power Determination: 21-23 Different concentrations of plant extract and standard ascorbic acid solution viz. 10, 20, 40, 60, 80, 100 μg/ml in 1 ml of methanol were mixed with phosphate buffer (2.5 ml, 0.2M pH 6.6) and potassium ferric cyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was incubated at 50 °C for 20 min. A portion (2.5 ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 g (rpm) for 10 min at room temperature.
The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml), and ferric chloride (FeCl3) (0.5 ml, 0.1%) and the absorbance of the reaction mixture indicated increased reducing power. The absorbance was measured at 700 nm. All the tests were performed in triplicate, and the graph was plotted with an average of three observations. Table 6 and Fig. 6
RESULTS AND DISCUSSION:
Identification and Authentication of Rivea ornata Roxb: Fresh and dry plants of Rivea ornata were collected from, Tirupati the month of May and its authentication was confirmed by Botanist, Dr. Madhava Chetty, S.V. University Tirupati.
Herbarium of the plant has been deposited at Department of Pharmacognosy, B. Pharmacy College, Rampura, Kakanpura, Panchmahal, Gujarat, India for future reference VJS/SD-35. The plant was further subjected to morphological and microscopically examination to access the purity of the procured plant drug and various diagnostic features were recorded for identification.
Proximate Analysis:
TABLE 1: STUDY OF DIFFERENT PARAMETERS OBTAINED FROM PROXIMATE ANALYSIS OF AERIAL PARTS OF RIVEA ORNATA ROXB.
| S. no. | Determination | Percentage w/w |
| 1 | Total ash | 15.45 |
| 2 | Acid-insoluble ash | 6.63 |
| 3 | Water soluble ash | 3.24 |
| 4 | Alcohol soluble extractive value | 4.69 |
| 5 | Water-soluble extractive value | 7.15 |
| 6 | Moisture content | 70.86 |
Phytochemical Studies:
Preliminary Phyto-profile: The percentage of different chemical constituents in the crude drug can be detected by subjecting them to successive extraction using solvents in the order of increasing polarity. The extract obtained was then dried completely and kept in vacuum desiccators. They were then subjected to qualitative chemical tests to detect the various chemical constituents present in them.
Tests for Preliminary Phytochemical Screening of Powder of Aerial Part of Rivea ornata Roxb.: Qualitative chemical examination of various successive extracts of powder indicated the presence of carbohydrates, steroids, triterpenoid glycosides, alkaloid, phytosterols, steroids, mucilage. Phytosterols were detected by Libermann Burchard test and Salkowaski reaction, carbohydrates by Molisch’s, Fehling’s and Benedict’s test, saponin by foam test, flavonoids by shinoda test, tannins and phenolics by lead acetate test.
Isolation and Identification of a Phenolic Compound from the Aerial Parts of Plant Rivea ornata: The methanol extract of Rivea ornata prepared by successive solvent extraction technique was used to isolate bergenin.
TLC Profile of Test Solution of Rivea ornata Roxb.: 10 μl of the methanol extract was spotted on TLC plate. It was developed in toluene: ethyl acetate: acetone (2:4:4) mobile phase. The plate was observed under UV light at 254 nm and after spraying with vanillin-sulphuric acid and heating at 100 °C till the colored bands appear.
Identification of the Isolated Phenolic Compound: The compounds C1 isolated at Rf – 0.74 showed positive chemical tests for phenolic compound (vaniline sulphuric acid). The melting point of C2 was 232-234 °C. Its structure was confirmed by different analytical techniques like UV-spectroscopy, IR-spectroscopy, LC-MS spectroscopy, and NMR-spectroscopy. For final confirmation UV spectra of compound C1 was taken with standard bergenin.
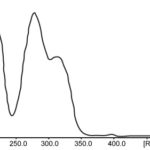
UV- Spectroscopy: The ultra violet spectra of compounds C1 was taken in methanol. The UV-spectra of compounds C1 was shown in Fig. 1 respectively.
TABLE 2: PRELIMINARY PHOTO PROFILE OF AERIAL PARTS OF RIVEA ORNATA ROXB.
| S.
no. |
Solvent | Color and consistency
after drying |
The average value (%w/w) |
| 1 | Petroleum ether (60 - 80ºC) | A yellowish, solid mass | 2.50 |
| 2 | Ethyl acetate | The greenish, sticky mass | 3.21 |
| 3 | Chloroform | The greenish, sticky mass | 2.13 |
| 4 | Methanol | Greenish yellow, sticky mass | 6.21 |
| 5 | Water | Dark Brown solid mass | 9.61 |
TABLE 3: TEST FOR PRELIMINARY PHYTOCHEMICAL SCREENING OF AERIAL PARTS OF RIVEA ORNATA ROXB.
| S.
no. |
Tests of
Phytoconstituents |
P. ether extract | Ethyl acetate extract | Chloroform extract | Methanol Extract | Water Extract |
| 1 | Tests for alkaloids
Mayer’s reagent Dragendorff’s reagent Hager’s reagent Wagner’s reagent |
* * * * |
* * * * |
+ve +ve +ve +ve |
+ve +ve +ve +ve |
+ve +ve +ve +ve |
| 2 | Tests for flavonoids
Shinoda test Fluorescence test FeCl3 test Lead acetate test |
* * * * |
* * * * |
* * * * |
-ve -ve -ve -ve |
-ve -ve -ve -ve |
| 3 | Tests for saponins
Froth test Hemolytic zone |
* * |
* * |
* * |
+ve +ve |
+ve +ve |
| 4 | Tests for carbohydrates
Molisch’s test Fehling’s solution test Benedict’s test: |
* * * |
* * * |
* * * |
+ve +ve +ve |
+ve +ve +ve |
| 5 | Tests for cardiac glycoside
Legal’s test Keller Killiani’s test Baljet test |
* * * |
* * * |
* * * |
-ve -ve -ve |
-ve -ve -ve |
| 6 | Tests for fixed oil and fat
Spot test Saponification test |
+ve +ve |
+ve +ve |
* * |
* * |
* * |
| 7 | Tests for sterols and triterpenoids
Libermann-Burchard's test Salkowski reaction |
+ve +ve |
* * |
* * |
* * |
* * |
| 8 | Tests for anthraquinone glycosides
Borntrager’s test Modifying borntrager’s test |
* * |
* * |
* * |
-ve -ve |
-ve -ve |
| 9 | Tests for phenolic compounds
Test with FeCl3 Test with folin-ciocalteu reagent |
* * |
* * |
* * |
+ve +ve |
+ve +ve |
| 10 | Tests for coumarins
With ammonia With hydroxylamine hydrochloride |
* * |
* * |
* * |
-ve -ve |
-ve -ve |
| 11 | Tests for tannins
Test with gelatin Reaction with lead acetate |
* * |
* * |
* * |
+ve +ve |
+ve +ve |
FIG. 1: ULTRAVIOLET SPECTRUM OF COMPOUND C1
Interpretation:
| Sample ID | λmax |
| Compound-C1 | 277 nm |
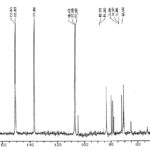
MS Spectroscopy: The MS spectra of compounds C1 was taken. The MS spectra of compounds C1 is shown in Fig. 2, Table 4 respectively.
FIG. 2: MS SPECTRUM OF COMPOUND C1
TABLE 4: INTERPRETATION
| Sample ID | m/z | Fragments |
| Compound- C1 | 121
208 235 269 326 |
[MH]
[MH-H2O] [C16H15N2O] [C15H15N2] |
The signals of the mass spectrum and their interpretation are consistent with the molecular formula of the compound.
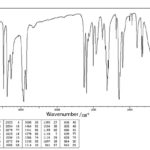
IR-Spectroscopy: The Infrared spectrum of compound C1 was taken. The IR-spectrum of compound C1 is shown in Fig. 3 and Table 5.
FIG. 3: INFRARED SPECTRUM OF COMPOUND C1
Interpretation: From the data obtained by FTIR, it could be concluded that the following functional groups are present.
TABLE 5: DATA FOR IR SPECTRUM OF COMPOUND C1
| Functional
Group |
Sample Peak
(cm-1) |
Standard Peak (cm-1) |
| O-H stretch | 3396 | 3200-2500 |
| C-H stretch (alkanes) | 2936 | 3200-2500 |
| C=O stretch | 1710 | 1735-1750 |
| C=C stretch | 1634 | 1620-1680 |
| C=C stretch | 1610 | 1620-1680 |
| C-O-C | 1363 | 1200-1320 |
| C-C stretch | 1076 | 1000-1260 |
| C-C(=O)-O | 1022 | 1000-1300 |
NMR - Spectroscopy: The 1H-NMR and 13C-NMR spectra of compound C1 were taken. The 1H-NMR and 13C NMR spectra of compound C1 are shown in Fig. 4 and Table 6 respectively.
H-NMR of Compound 1
FIG. 4: H-NMR AND C-NMR OF COMPOUND 1
TABLE 6: 1H NMR AND 13C NMR SPECTRAL DATA OF COMPOUND 1 (CD3OD)
| Position | 1H NMR
(40MHz) |
13C NMR
(100 MHz) |
| 1 | 118.6 | |
| 2 | 107.6 | |
| 3 | 144.9 | |
| 4 | 151.4 | |
| 5 | 150.8 | |
| 6 | 7.13 (1H, s) | 107.5 |
| 7 | 167.8 | |
| 1′ | 4.86 (1H, d,
J = 10.3 Hz) |
74.6 |
| 2′ | 3.78 (1H, m) | 75.8 |
| 3′ | 3.95 (1H, m) | 81.4 |
| 4′ | 3.44 (1H, m) | 71.8 |
| 5′ | 3.62 (1H, m) | 82.8 |
| 6′ | 3.68 (1H, m) | 62.7 |
| 4.00 (1H, m) | ||
| OCH3 | 3.81 (3H, s) |
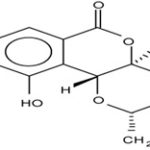
Compound C1 was isolated as white crystals. UVmax (MeOH): 275 nm; IR bands (KBr): 3425, 2885, 2724, 1702, 1614, 1528, 1464, 1421, 1375, 1341, 1233, 1093 and 1046 cm−1. The mass spectral data of the compound gave a molecular formula C14H16O9, m/z 328 for M+. 1H NMR and C NMR were shown above.
From the spectral data, it was concluded that the compound C1 was Bergenin:
FIG. 5: STRUCTURE OF BERGENIN
HPTLC Finger-Printing and Quantitative Determination of Isolated Bergenin: CAMAG TLC scanner 3 and LINOMAT-V densitometry evaluation system with WINCAT software was used for scanning of thin layer chromatogram objects in reflectance or transmission mode by absorbance or by fluorescence at 254 or 365 nm, respectively.
Rf value of the sample was evaluated using the following formula. Rf = Distance traveled by sample from baseline Distance traveled by solvent from baseline calibration of Bergenin.
Different concentration (1μg/ml - 5μg/ml) using micro syringe from the standard solution of 1000 mcg/ml were spotted as sharp band of 5 mm width on precoated silica gel aluminium plate 60F254, (10 × 10 cm) using toluene: ethyl acetate: acetone (2:4:4).
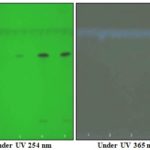
FIG. 6: HPTLC PLATS OF BERGENIN
Track-1: 1 μg/ml of Standard bergenin. Track-2: 2 μg/ml of Standard bergenin. Track-3: 3 μg/ml of Standard bergenin. Track-4: 4 μg/ml of Standard bergenin. Track-5: 5 μg/ml of Standard bergenin
FIG. 7: DENSITOMETRY CHROMATOGRAM OF BERGENIN STANDARD
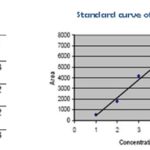
FIG. 8: CALIBRATION CURVE OF STANDARD BERGENIN
The peak areas of bergenin for (1μg/ml-5μg/ml) concentration were recorded. A calibration curve was prepared by plotting peak areas of bergenin against concentration Fig. 8. The results of the linearity range and correlation coefficient showed that within the concentration (1μg/ml-5μg/ml) range indicated; there was a good correlation between peak area and the corresponding concentration of bergenin. The best fitting line equation was y = 1695.1X - 1276.7.
HPTLC of Bergenin with Rivea ornata Roxb:
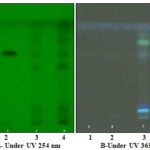
FIG. 9: HPTLC PLATES OF RIVEA ORNATA ROXB.
Track-1: 2 μg/ml of standard bergenin. Track-2: 4 μg/ml of standard bergenin. Track-3: 2 μg/ml of Methanol extract of Rivea ornata Roxb. Track-4: 4 μg/ml of Methanol extract of Rivea ornata Roxb.
FIG. 10: DENSITOMETRY CHROMATOGRAM OF RIVEA ORNATA ROXB.
TABLE 7: HPTLC FOR BERGENIN IN RIVEA ORNATA ROXB.
| Track | Peak | Start Rf | Start Ht. | Max Rf | Max Ht. | End Rf | Area | Bergenin mcg/ml |
| Bergenin | 1 | 0.71 | 33.2 | 0.74 | 43.4 | 0.76 | 867.4 | - |
| Methanolic extract
R. ornata Roxb. |
1 | 0.72 | 0.6 | 0.74 | 19.1 | 0.76 | 1470.5 | 0.331 |
Silica gel TLC plate as the stationary phase and toluene: ethyl acetate: acetone (2:4:4) as the mobile phase gives good separation of Bergenin at Rf -0.74. The HPTLC photographed complete is shown in Fig. 6. The detector response/calibration curve of Bergenin was found to be linear dependent on the concentration against the area. The best fitting line equation was y = 1695.1X-1276.7. Correlation coefficient 0.989 indicates good linearity between concentration and peak area in Fig. 8. The concentration of Bergenin in the methanolic extract of dried leaves powder of Rivea ornata Roxb. by proposed HPTLC method was found to be 0.331 mcg/ml. The identity of the Bergenin band in the sample extract solution was confirmed by overlaying/superimposing the UV absorption spectrum of the sample with that from the reference standard of bergenin, using the Camag TLC scanner 3 Fig. 9, 10 and Table 7.
DPPH Method:
TABLE 8: 1, 1-DIPHENYL-2-PICRYL HYDRAZYL (DPPH) RADICALS SCAVENGING ACTIVITY
| S.
no. |
Concentration
µg/ml |
% inhibition | |
| Alcoholic extract | Ascorbic acid | ||
| 1 | 0 | 0 | 0 |
| 2 | 10 | 43.84 | 45.56 |
| 3 | 20 | 50.2 | 55.5 |
| 4 | 40 | 58.5 | 60.33 |
| 5 | 60 | 60.59 | 47.65 |
| 6 | 80 | 61.96 | 70.22 |
| 7 | 100 | 65 | 75.15 |
| 8 | IC50 value | 21.2 | 11.33 |
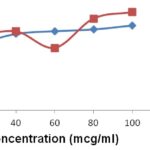
FIG. 11: 1, 1-DIPHENYL-2-PICRYL HYDRAZYL (DPPH) RADICALS SCAVENGING ACTIVITY % INHIBITION vs. CONCENTRATION
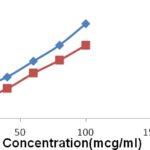
Antioxidant activity of the plant Rivea ornata Roxb.: The antioxidant activity of the alcoholic extract of the plant Rivea ornata Roxb. was carried out by in-vitro antioxidant models. In the models tested, the antioxidant activity of the formulation was studied about Ascorbic acid, a known antioxidant. The result indicated the significant decrease in the concentration of DPPH radicals due to the scavenging ability of Alcohol extract of plant Rivea ornata Roxb. and ascorbic acid, as a reference standard. Maximum inhibition of extract and ascorbic acid was exhibited 65.0% and 75.15% inhibition respectively in 100 μg/ml. The IC50 values in DPPH radical scavenging model were 11.33 μg/ml and 21.2 for Ascorbic acid and alcohol extract of plant Rivea ornata Roxb. respectively.
Ferric Reducing Antioxidant Power: The result illustrates that Alcoholic extract of the plant Rivea ornata Roxb. had ferric reducing capacity and also comparable to ascorbic acid.
TABLE 8: FERRIC REDUCING ANTIOXIDANT ACTIVITY
| Ferric reducing power | ||
| Concentration
(mcg/ml) |
Absorbance | |
| Ascorbic
acid |
Alcoholic extract | |
| 0 | 0 | 0 |
| 10 | 0.05 | 0.024 |
| 20 | 0.08 | 0.048 |
| 40 | 0.11 | 0.079 |
| 60 | 0.152 | 0.12 |
| 80 | 0.195 | 0.155 |
| 100 | 0.252 | 0.195 |
FIG. 12: FERRIC REDUCING ANTIOXIDANT ACTIVITY ABS vs. CONC.
CONCLUSION: Rivea ornata Roxb. (syn. Phang), family Convolvulaceae, is a woody climber, occurring in south India in Tripura. Rivea ornata Roxb. leaves contain total ash (13.80%), acid insoluble ash (7.52%), water soluble ash (6.21%), alcohol soluble extractive value (6.58%), water soluble extractive value (7.69%) and moisture content (82.65%). Petroleum ether ((60 - 80 ºC) extract of Rivea ornata Roxb. was yellowish mass (2.53% w/w), toluene extract was green sticky mass (3.12% w/w), chloroform extract was greenish yellow sticky mass (2.13% w/w), methanol extract was greenish brown sticky mass (3.21% w/w) and water extract was reddish brown sticky mass (8.24% w/w).
Compound C2 was isolated as white crystals. UVmax (MeOH): 277 nm; IR bands (KBr): 3425, 2885, 2724, 1702, 1614, 1528, 1464, 1421, 1375, 1341, 1233, 1093 and 1046 cm−1. The mass spectral data of the compound gave a molecular formula C14 H16O9, m/z 328 for M+.1H NMR (DMSO-d6, 400 MHz): δ 6.48 (m, arom., H-7), 5.68 (1H, d, H-10b), 4.99 (1H, dd, H-4a), 3.99 (1H, dd, H-4), 3.80 (2H, d, H-11), 3.76 (3H, s, H-12), 3.60 (1H, m, H-2), 3.49 (1H, dd, H-3). 13C NMR (DMSO- d6, 100 MHz): δ 60.0 (C-12), 61.2 (C-11), 70.8 (C-3), 72.2 ( C-10b), 73.8 (C-4), 79.9 (C-4a), 81.8 (C-2), 109.6 (C-7), 116.1 (C-10a), 118.2 (C-6a), 140.7 (C-9), 148.2 (C-10), 151.1 (C-8), 163.5 (C-6). From the spectral data it was concluded that the compound C1 was Bergenin.
Qualitative chemical examination of various extracts of Rivea ornata Roxb. was carried out which revealed the presence of phytoconstituents like carbohydrates, phytosterols, phenolic compounds, alkaloid, triterpenoids, fixed oil, and tannins.
The reducing power of Rivera ornata Roxb. extracts increased steadily with increasing concentrations and varied significantly with different concentrations. The methanol and ethyl acetate extracts appeared to possess the highest significant reducing activity among the extracts. The stronger reducing power in the methanol and ethyl acetate extracts was probably due to the concentration of antioxidant compounds like flavonoids and phenolics in the extract. In conclusion, the antioxidant study of Rivea ornata Roxb. suggested that Rivea ornata Roxb. is a potential source of natural antioxidants. However, further investigations in-vivo antioxidant activities are highly recommended. It is also needed to determine phytoconstituents, which are responsible for the antioxidant activity.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Medicinal plants in tropical countries, Markus S. Mueller; 2005
- http://www.umm.edu/altmed/articles/herbal-medicine-000 351.htm
- Han LK, Ninomiya H, Taniguchi M, Baba K, Kimura Y, and Okuda H: Norepinephrine-augmenting lipolytic effectors from Astilbe thunbergii Journal of Natural Products 1998; 61(8): 1006-11.
- Kim HS, Lim HK, Chung MW and Kim YC: Anti-hepatotoxic activity of bergenin, the major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated hepatocytes. J of Ethnopharmacology 2000; 69(1): 79-83.
- Abe K, Sakai K and Uchida M: Effects of bergenin on experimental ulcers–prevention of stress-induced ulcers in rats. General Pharmacology 1980; 11(4): 361-8.
- Goel RK, Maiti RN, Manickam M and Ray AB: Antiulcer activity of naturally occurring pyrano-coumarin and isocoumarins and their effect on prostanoid synthesis using human colonic mucosa. Indian Journal of Experimental Biology 1997; 35(10): 1080-3.
- Nazir N, Koul S, Qurishi MA, Taneja SC, Ahmad SF, Bani S and Qazi GN.: Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis–a flow cytometric study. Journal of Ethnopharmacology. 2007; 112(2): 401-5.
- Ye YP, Sun HX and Pan YJ.: Bergenin monohydrate from the rhizome of Astilbe chinensis. Acta Crystallographica. Section C, Crystal Structure Communications 2004; 60(Pt): 397-8.
- Singh U, Barik A and Priyadarsini KI: Reactions of hydroxyl radical with bergenin, a natural polyphenol studied by pulse radiolysis. Bioorg Med Chem 2009; 17: 6008-6014.
- Quality controls methods for medicinal plant materials. World Health Organization. Delhi: Geneva AITBS Publisher and Distributors, 2002; 28-30, 38-40, 64-73.
- Indian Pharmacopoeia: The Controller of Publication; Delhi, 1996, 2: A- 95,736 and A-81-83.
- Anonymous: British Pharmacopoeia". London: HMSO; Vol. 1, 1993: 222.
- Kokate CK, Purohit AP and Gokhale SB: Pharmacognosy. Pune: Nirali Prakashan, Edition 36th, 2006: 593-97.
- CK Kokate: Practical Pharmacognosy" Delhi: Vallabh Prakashan, Edition 4th, 1994: 148.
- Hussain A, Wahab S, Rizvi A and Hussain MS: Macroscopical, anatomical andphysicochemical studies on leaves of Coccinia indica Wight & Arn. Indian J Nat Prod Resour 2011; 2(1): 74-80.
- Mukharji P: Quality Control of Herbal Drugs. New Delhi: Business Horizons Pharmaceutical Publishers, Edition 1st, 2002: 708-10.
- Hoffmann A: The active principle of the seed of Rivea corymbosa and Ipomea violacea. Botanical museum leaflet, Harvard University, Vol. 2, 1962; 64: 194-212.
- Taneyama M, Yoshida S, Kobayashi M and Hasegawa M: Isolation of norbergenin from Saxifraga stolonifera. Phytochemistry 1983; 22: 1053-1054.
- Sharma J and Fried B: Handbook of Thin-Layer Chromatography. Revised and Expanded. New York, Bsel: Marcel Dekker, Inc., Edition 3rd, 1989: 82-87.
- OECD Guideline (425) for the testing of chemicals: Guidance document on acute oral toxicity, Environmental Health and Safety Monograph Series on Testing and Assessment, 2000.
- John A and Steven DA: Microsomal lipid peroxidation, Methods in Enzymol 1984; 30: 302-308.
- Gupta M, Mazumdar UK, Gomathi P and Sambath RK: Antioxidant and free radical scavenging activities of Ervatamia coronaria Leaves. Iranian Journal Pharm Research 2004; 2: 119-126.
- Srinivasan R, Chandrasekar MJN, Nanjan MJ and Suresh B: Free Radical scavenging activity of obscura (L.) Ker-Gawl. J of Natural Remedies 2007; 7(2): 184-188.
How to cite this article:
Sharma VJ and Patel PM: Bergenin – an active constituent of Rivea ornata Roxb. and its antioxidant property. Int J Pharmacognosy 2017; 4(9): 309-19. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.4(9).309-19.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
6
309-319
866
1555
English
IJP
V. J. Sharma * and P. M. Patel
Department of Pharmacognosy, B. Pharmacy College Rampura Kakanpur, Godhra, Panchmahal, Gujarat, India.
vaishalisharma84@gmail.com
29 May 2017
08 July 2017
11 July 2017
10.13040/IJPSR.0975-8232.IJP.4(9).309-19
01 September 2017