BENZOQUINONE MONO OXIMES DERIVATIVES WITH ANTICANCER ACTIVITY
HTML Full TextBENZOQUINONE MONO OXIMES DERIVATIVES WITH ANTICANCER ACTIVITY
Chistiane Mendes Feitosa * 1, Sosthenes de Sousa Silva 1, Gardenia Gadelha Carmem Militão 2, Damião Pergentino de Sousa 3, Khaled Rashed 4 and Layana Karine Farias Lima 5
Post-graduate Programs in Chemistry and Pharmaceutical Sciences 1, Federal University of Piaui,Ininga, 64.049-550, Teresina, Piauí, Brazil.
Department of Physiology and Pharmacology 2, Federal University of Pernambuco, 50670-901, Recife, PE, Brazil.
Departamento de Ciências Farmacêuticas 3, Universidade Federal da Paraiba, Campus I, Cidade Universitária, 58051900, João Pessoa-Paraiba, Brasil.
Department of Pharmacognosy 4, National Research Centre, 33 El-Bohouth st.-Dokki, Giza, Egypt- P.O.12622.
Laboratory of Natural Products and Experimental Neurochemistry 5, Federal University of Piaui, Ininga, 64.049-550, Teresina, Piauí, Brazil.
ABSTRACT: Objective: The aim of this study was to evaluate the anticancer activity of a series of six structurally related [1, 4]-Benzoquinone monooximes and to investigate structure-activity relationships (SAR) of these compounds. Materials and Method: The mono-oximes obtained by oximination of para-benzoquinones 1-4 had their structures confirmed by ¹H NMR, 13C NMR. The cytotoxic study was assessed by the MTT assay on three tumor cell lines: HL60 (promyelocytic leukemia), NCI-H292 (lung cancer), and MCF-7 (breast adenocarcinoma). Results: Six oximes were synthetized 2 - methyl-[1,4]-benzoquinone oxime tosylate (I), 5-isopropyl-2-mehyl-[1,4]-benzoquinone oxime tosylate (II), 5-isopropyl-5-mehyl-[1,4]-benzoquinone oxime tosylate (III), 5-isopropyl-2-mehyl-[1,4]-benzoquinone oxime (IV), 2,5-dimethyl-[1,4]-benzoquinone oxime tosylate (V), 2-methyl-[1,4]-benzoquinone oxime tosylate (VI). Compounds II to V showed significant activity against all cell lines; while compound I and VI presented low or no activity. Conclusion: These experimental data lead to the conclusion that oximes (II –V) may be used as potential starting structures for the design of novel anticancer agents.
| Keywords: |
Oximes, Cytotoxic, Anticancer
INTRODUCTION: Cancer figures among the leading cause of death worldwide, and it is expected that the annual cancer cases will rise to 22 million within the next two decades, according to the World Health Organization (WHO) 1. The cancers that are well diagnosed are skin cancer, prostate, female breast, colorectal, lung, stomach, and cervical.
Cancer has been considered a major public health problem 2. According to the National Institute of Cancer, it was estimated for the biennium 2016-2017, 600 thousand new cases in Brazil 3.
Oximes contained secondary metabolites that constitute an important group of bioactive compounds and have been described and frequently updated in the literature due to their pharma-cological properties, such as antioxidants 4 acetylcholinesterase inhibitors 5 and antitumor 6. Oximes are substances that have been investigated for many years as compounds with high potential in the treatment against poisoning, including, for example, organophosphates (OPs), including pesticides and nerve agents; however, currently, few oximes have clinical application. One of the purposes of its use involves the reactivation of acetylcholinesterase (AchE) enzyme; however, still has disadvantages and limited action in the reactivation of this enzyme 7, 8. However, the limitation of conventional oximes has encouraged the synthesis of new oximes with various structural modifications 9. Oxidative stress is the imbalance between antioxidant defenses and free radicals and reactive metabolites, so-called oxidants or reactive oxygen species (ROS) that under prolonged stress condition scan induce neoplasm tictrans formations 10. Major research suggests that the oximes acted decreasing lipid peroxidation after poisoning with malathion, hyperstimulation experiment sin the cholinergic system, and oxidative stress in the prefrontal cortex of rats 4. In promising research oximes synthesized as follows: paraoxon, pralidoxime, thiophene - 2 - aldoxime, 4-methoxy-benzaldoxime, benzaldoxime, 4 - Fluor -benzaldoxime, 4-chloro-benzaldoxime and 4-nitro-benzaldoxime showed potent inhibition action AchE and promise for the future development of drugs for alzheimer's disease 5. Oxime tosylate (I), 5-isopropyl-2-mehyl-[1,4]-benzoquinone oxime to sylate (II), 5 – isopropyl – 5 – mehyl - [1, 4]-benzo-quinone oxime tosylate (III), 5-isopropyl-2-mehyl-[1,4]-benzoquinone oxime (IV), 2, 5-dimethyl-[1,4]-benzoquinone oxime tosylate (V), 2-methyl-[1,4]-benzoquinone oxime tosylate (VI) and others oxime derivatives are reported with larvicidal activity against Aedes aegypti L. 11. The (4E) oxime of 2 - isopropyl - 5 methyl - para -benzoquinone and the corresponding 2-diethyl-amino-ethyl derivative were reported with antidepressant activities potent 12. Thus, the aim of this study was to evaluate the cytotoxic activity of a series of six structurally related [1, 4]-Benzo-quinone mono oximes and to investigate structure-activity relationships (SAR) of these compounds. The cytotoxic study was conducted by the method salt 3-(4, 5-dimethyl-2-thiazole)-2, 5-diphenyl-2 H-tetrazolium bromide (MTT), on tumor cell lines: HL60 (leukemia pro-myelocytic), NCI-H292 (lung cancer) and MCF-7 (breast cancer).
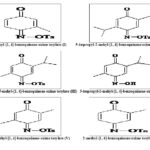
FIG. 1: BENZOQUINONE MONO OXIMES DERIVATIVES COMPOUNDS
MATERIALS AND METHODS:
General Procedure for the Synthesis of the 1, 4-Benzoquinone Mono-oximes (I-VI): The phenol (73.5 mmol) was dissolved in 10 M hydrochloric acid (50 ml) and 95 % ethanol (50 ml). Sodium nitrite (7.5 g, 108.7 mmol) was added at 0 °C for over 5 min with stirring, and the reaction mixture was then stirred for an hour at 0 °C. Ethanol (10 ml) was then added, and stirring continued for a further hour at room temperature. The reaction mixture was diluted with water (500 ml) and extracted with diethyl ether. The ethereal extract was then washed with 10% aqueous sodium carbonate solution. The aqueous solution, on acidification with 3 M hydrochloric acid, yielded a dirty yellow precipitate which was filtered. The solid residue was washed with hexane to eliminate soluble impurities to give the product (I –VI).
Preparation of the Oxime Derivatives: 1H and 13C NMR spectra were obtained on a Bruker DRX400 spectrometer at 400 and 100 MHz, respectively, with CDCl3 as a solvent.
Chemical shifts are reported in ppm downfield fromate tramethylsilane internal standard. Infrared spectra were recorded on a Bomen Michelson model 102 FTIR or a Hartman & Braun MB, and the most intense or representative bands are reported (in cm−1).
Melting points were determined ona Micro Quí-micamodel APF 301 apparatus and are uncorrected. Solvents and reagents were used directly from the supplier or purified when required by standard procedures.
Compound I: 80% Yield: 1HRMN (CDCl3, 400 MHz) δ: 2.13 (CH3, d, J=1.3 Hz); 2.47 (CH3, s); 6.33 (CH, m); 6.45 (CH, dd, J=10.3 Hz, 2.0 Hz); 7.38 (CH, d, J=8.3 Hz); 7.57 (CH, d, J=10.3 Hz); 7.91 (CH, d, J=8.3 Hz). 13CRMN (CDCl3, 100 MHz) δ: 17.2; 21.7; 124.8; 129.2; 129.8; 131.0; 131.4; 133.6; 145.0; 146.1; 153.6; 186.1. IR (νmax., KBr, cm-1): 2974, 1649, 1593, 1386, 1196, 1175, 1092.
Compound II: 80% Yield: 1HRMN (CDCl3, 400 MHz) δ: 1.07 (CH3, d, J=7.1 Hz); 2.01 (CH3, d, J = 1.5 Hz); 2.45 (CH3, s); 3.08 (CH, d hept, J= 0.7 Hz, 7.1 Hz); 6.31 (CH, d, J=0.7 Hz); 7.36 (CH, J=8.3 Hz); 7.40 (CH, q, J= 1.5 Hz); 7.89 (CH, J=8.3 Hz). 13C RMN (CDCl3, 100 MHz) δ: 15.8; 21.7; 21.9; 27.4; 121.6; 127.3; 129.1; 129.7; 131.7; 142.3; 145.8; 152.4; 154.5; 186.8. IR (νmax., KBr, cm-1): 2958, 1654, 1386, 1195, 1176, 1091.
Compound III: 79% Yield: 1H RMN (CDCl3, 400 MHz) δ: 1.10 (CH3, d, J=6.8 Hz); 2.08 (CH3, d, J=1.5 Hz); 2.46 (CH3, s); 3.04 (CH, d hept, J=1.3 Hz, 6.8 Hz); 6.30 (CH, q, J=1.5 Hz); 7.27 (CH, d, J=1.3 Hz); 7.37 (CH, d J=8.3 Hz); 7.92 (CH, d, J=8.5 Hz). 13C RMN (CDCl3, 100MHz) δ: 16.7; 21.5; 21.7; 26.9; 118.2; 129.2; 129.7; 131.4;.131.7; 144.2;.145.8; 152.2;.154.0;.185.6. IR (νmax., KBr, cm-1): 2964, 1647, 1599, 1382, 1195, 1181, 1095.
Compound IV: 65% Yield: 1H RMN (CDCl3, 400 MHz) δ: 1.13 (CH3, d, J=6.8 Hz); 2.20 (CH3, d, J=1.3 Hz); 3.06 (CH, d hept, J=1.2 Hz, 6.8 Hz); 6.26 (CH, q, J=1.3 Hz); 7.56 (CH, d, J=1.2 Hz). 13C RMN (CDCl3, 100MHz) δ: 16.9; 21.5; 26.4; 118.4; 128.5; 146.2; 147.9; 150.2; 186.9. IR (νmax., KBr, cm-1): 3480, 2961, 1638, 1604, 1439, 1241, 1055 Mp 142.6-143.2 ºC.
Compound V: 88% Yield: 1H RMN (CDCl3, 400 MHz) δ: 2.00 (CH3, d, J=1.5 Hz); 2.09 (CH3, d, J=1.2 Hz); 2.45 (CH, s); 6.30 (CH, q, J=1.2 Hz); 7.36 (CH, d, J=8.3 Hz); 7.37 (CH, q, J=1.5 Hz); 7.90 (CH, d, J=8.3 Hz). 13C RMN (CDCl3, 100 MHz) δ: 15.9; 19.8; 21.7; 121.3; 129.1; 129.8; 130.9; 131.7; 142.8; 144.8; 145.9; 153.9; 186.4. IR (νmax., KBr, cm-1): 2926, 1654, 1602, 1377, 1191, 1176, 1091.
Compound VI: 85% Yield: 1H RMN (CDCl3, 400 MHz) δ: 2.02 (CH3, d J=1.5 Hz); 2.07 (CH3, d J=1.5 Hz); 2.48 (CH3, s); 6.53 (CH, d, J=10.1 Hz); 6.54 (CH, d, J=10.1 Hz); 6.96 (CH, m); 7.09 (CH, dd, J=10.1 Hz, 2.5 Hz); 7.38 (CH, d, J=8.5 Hz); 7.40 (CH, d, J=8.5 Hz); 7.46 (CH, m); 7.57 (CH, dd, J=10.1 Hz, 2.5 Hz); 7.91 (CH, d, J=8.5 Hz); 7.93 (CH, d, J=8.5 Hz). 13C RMN (CDCl3, 100 MHz) δ: 15.7; 16.4; 21.7; 120.9; 124.1; 129.0; 129.1; 129.8; 131.1; 131.5; 132.4; 132.5; 134.3; 135.1; 145.9; 153.4; 153.6; 186.3; 186.4. IR (νmax., KBr, cm-1): 2926, 1641, 1634, 1387, 1193, 1176, 1091.
Antiproliferative Activity: The human tumor cell lines used in this work were HL-60 (promyelocytic leukemia), MCF-7 (breast carcinoma), NCI-H292 (lung carcinoma), and these cells were obtained from Rio de Janeiro Cell Bank (RJ, Brazil). Cancer cells were maintained in RPMI 1640 medium or DMEN supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin at 37 °C with 5% CO2. We assessed the cytotoxicity of the compounds against four tumor cell lines using the 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H tetrazolium bromide (MTT) (Sigma Aldrich Co., St. Louis, MO, USA) reduction assay.
For all experiments, tumor cells were plated in 96-well plates (105 cells/mL for adherent cells or 3 x 105 cells /mL for leukemia) 13. After 24 h, compounds dissolved in DMSO 0.5% were added to the wells at 25 μg/ml for initial screening. Compounds that presented over 75% cell growth inhibition were considered to be active. Further, active compounds were tested in serial dilutions (0.3-25 µg/mL). Control groups received the same amount of DMSO. After 69 h of treatment, 25 µL of MTT (5 mg/mL) was added, three hours later, the MTT formazan product was dissolved in 100 μL of DMSO, and absorbance was measured at 595 nm in plate spectrophotometer.
Doxorubicin (0.01–5 µg/mL) was used as positive control. IC50 values and their 95% confidence intervals for two different experiments were obtained by non linear regression using Graphpad Prism version 5.0 for Windows (GraphPad Software, San Diego, California, USA) 14, 15.
RESULTS AND DISCUSSION: In the present investigation, six analogs of the [1, 4] benzoquinone oxime were synthesized and subjected to cytotoxic bioassay on tumor cell lines: HL-60 (promyelocytic leukemia), MCF-7 (breast adenocarcinoma) and NCI-H292 (lung carcinoma). In addition, the structure-activity relationship (SAR) was investigated. The synthesis of the [1, 4] benzoquinone oxime compounds Fig. 1 and structural determination of these compounds are consistent with those described by 11. The parameter is most commonly used to evaluate the cytotoxicity and cell viability, which can be detected by the technique of vital dyes such as neutral red, which allows the distinction between living cells and dead 16. In this study, cells were performed using the formazan dye (formed by the reduction of salt 3-(4, 5-dimethyl-2-thiazole)-2, 5-diphenyl-2H-tetrazolium bromide (MTT). In this colorimetric method, the activity of viable cells with active metabolism converts the salt MTT to the purple-coloreddye MTT-formazan, which can be quantified spectrophotometrically 17.
Inhibition of cell growth, an indicator of cytotoxicity caused by the compounds being valuated for antineoplastic activity in this study were visualizedin cells HL60 (promyelocytic leukemia), NCI-H292 (lung cancer) and MCF-7 (breast adenocarcinoma), however, other cells may be used to find out further, studies: Hep2 (ATCC -CCL-23, de carcinoma de laringe), B16-F10 (ATCC CRL-6322, células de melanoma), 786 (ATCC – CRL1932, carcinoma de rim), NCI-ADR (carcinoma de ovário resistente a adriamicina), OVCAR 5 3 (ATCC HTB 161, ovarian carcinoma), UACC-62 (melanoma), HT-29 (ATCC HTB-38, colon carcinoma), PC03 (ATCC CRL 1435, próstatacarcinoma). According to the International Standard Organization, ISO10993, the in-vitro cytotoxicity as say is the first test to evaluate the toxic effects in animal models; having proven its non-toxicity study may continue carrying out necessary tests in the animal laboratory.
In-vitro methods have advantages over in-vivo such as might limit the number of experimental variables, to obtain meaningful data more easily beyond the test period is in most cases, shorter 18. The problem of extrapolation of the data obtained in-vitro for the clinical application of biomaterial scan is overcome by the use of appropriate reference material currently used in clinics.
Studies using these methods showed that the tests with cell cultures could be used successfully as they are reproducible, fast, sensitive, and affordable for the implementation of in-vitro biocompatibility study 19.
The synthesized compounds were the screened at 25 µg. mL-1 against HL-60 (promyelocytic leukemia), MCF-7 (breast adenocarcinoma), NCI-H292 (lung carcinoma), tumor cells lineages. The samples with growth inhibition over 90% were used to determine the IC50 values (concentration that causes 50% growth inhibition). Analogs II, III, IV, V and VI exhibited good cytotoxicities, and their IC50 values are described in Table 1.
TABLE 1: CONCENTRATIONS THAT INHIBIT 50% CELL GROWTH (IC50) µg/ml AND CONFIDENCE INTERVALS (CI 95%) OF COMPOUNDS IN THREE TUMOR CELL LINES AFTER 72 H OF INCUBATION
| Compounds | HL-60 | MCF-7 | NCI-H292 |
| CI50 (Confidence Intervals) µg/mL | |||
| II | <0.3 | 3.6 (3.0 – 4.4) | 1.6 (1.4 – 1.9) |
| III | <0.3 | 3.1 (2.7 – 3.7) | 1.7 (1.5 – 1.8) |
| IV | 1.9 (1.6 – 2.2) | 14.8 (12.3 – 17.9) | 9.0 (7.7 – 10.5) |
| V | 0.3 (0.2– 0.3) | 5.9 (4.9 – 7.0) | NT* |
| VI | 4.1 (3.1 – 5.5) | 16.3 (8.5 – 31.1) | >25 |
| Doxorubicin | 0.02 (0.01-0.02) | 0.30 (0.20-0.50) | 0.01 (0.01-0.03) |
NT* Not Tested
When tested at single concentration of 25 µg/mL, compounds II to V were active at all cell lines, while compound VI was high active only at HL60 and compound I showed no activity (see Table 1 and 2).
TABLE 2: CELL GROWTH INHIBITION (IC %) OF COMPOUNDS ON THREE TUMOR CELL LINES
| Samples | Cell Lines (IC %) | ||
| HL60 | MCF-7 | NCI-H292 | |
| I | 0.0 | 0.0 | 0.0 |
| II | 97.2 ± 0.6 | 93.7 ± 0.8 | 96.1 ± 0.5 |
| III | 96.5 ± 2.6 | 88.1 ± 2.8 | 93.04 ± 5.5 |
| IV | 96.3 ± 3.0 | 81.4 ± 9.6 | 91.7 ± 7.2 |
| V | 99.1 ± 0.3 | 92.9 ± 2.2 | 96.1 ± 1.1 |
| VI | 91.0 ± 3.6 | 54.7 ± 13.0 | 42.4 ± 2.8 |
Derivatives were tested at 25 μg/ml on cells for 72 h of incubation.
In fact, compounds II and III, the most active ones, presented similar IC50 values ranging from concentrations below 0.3 µg/mL to 3.6 µg/mL. After performing the test for the determination of 50% inhibitory concentration for these compounds on tumor cells was observed IC50 values lower than 0.3 µg /mL for HL60 and IC50 ranging from 1.6 to 16.3 in the adhered cells (MCF-7 and NCIH292), demonstrating that the II, III, IV and VI compounds contain moderate to high cytotoxicity. Pure substances that had IC50< 1 µg /mL are considered very active 20, 21. According to the functional and structural characteristics and the observed cytotoxic activity, the tested compounds can be divided into two classes, para-benzoquinone oxime derivates with presence or absence of alkyl groups, white the second group involve tosylatedoxime derivatives with additional alkyl groups. To investigate if the introduction of a methyl group in the chemical structure influences the larvicidal activity, benzoquinoneoxime (I) was compared to benzo-quinone oxime (V). Compound (V) was more bioactive than (I) indicating tha the addition of a lipophilic group improves the activity 11, 22, 23 demonstrated that the presence of methyl groups in unsubstituted quinones promoted a significant increase of larvicidal activity against different mosquito species.
Compound IV and V showed to be moderated cytotoxic. Compound (VI) was low active. Replacement of a methyl at carbon 2 by an isopropyl group contributed to increase the citotoxic activity; 5- isopropyl- 2- mehyl-[1,4]-benzoquinone oxime tosylate (II) was more bioactive the 2, 5-dimethyl-[1, 4]-benzoquinone oxime (V) Searches a novel series of 4-aminopyrimidine-5-carboxaldehyde oxime that have activity against as antiproliferative VEGFR-2 inhibitor has been identified and structure–activity relationships (SAR). The best potency was achieved with 4-fluoro-2-methylindol-5-yloxy at the-6-position. On the oximeside chain, lipophi licalkyl groups we repreferred over hydrophilic amino sidechain 24. It can be seen the [3, 1]-benzo-thiazepine and [3, 1] benzoaxezepine derivatives displayed moderate cytotoxic activity against all tested câncer cell lines, being more active for HL60 cells 25.
One of these derivatives exhibited better anti-proliferative activity against HL-60 and HT-29 cell lines, being also more active for HL-60 with an IC50 = 2.1 µg mL-1., demon-strating the potential of the newly synthesized compounds as antitumoral agentes. Our results were more promising, II, III and IV oximes exhibited better anti-proliferative activity against HL-60 (< 2 µg/mL). A promising study was released with other benzoquinones as cytotoxic evaluation: cytotoxicity activity of analogues 3 and 6 alkyl-Substituted. 3- and 6-alkyl-2-methoxy-1, 4-benzoquinone derivatives ana-logues, Tests were performed to evaluate the cytotoxicity activity with continuous chain KB cells (epidermoide carcinoma of the floor of the mouth). In this series 6-methyl-2-methoxy-1, 4-benzoquinone was the most active that showed the highest inhibition on KB cells (IC50 = 0.27 µg/ml) 26. The compounds synthesize 5 - (pentyl) – 3 - (m-tolyl)- 1, 2, 4-oxadiazole, (p-bromophenyl)-5-(pentyl)-1,2,4-oxadiazol and p - chlorophenyl) – 5 - (pentyl) -1, 2, 4 - oxadiazol: was tested against three human cell lines (NCI H292; HL-60 and HT29) exhibited moderate anti-proliferative activity against HL-60 cancer cell lines with IC50 values of 42.1, 19.0 and 28 μM, respectively 27 II, III and IV oximes exhibited better anti-proliferative activity against HL-60 (< 2 µg/mL). In Table 1, the results of cytotoxicity of the compounds (I-VI) in comparison with oxorubic in anticancer drug, widely used as a chemotherapeutic drug, are listed. The oximes II to V demonstrated significant activity against all tested tumor cell lines, with a result above the control doxorubicin drug.
The best potency was achieved with 5 – isopropyl – 2 – mehyl - [1, 4]-benzoquinone oxime tosylateat the 5-isopropyl position (oxime II). Oxime (I) showed no cytotoxic action. Oximes (II, III, IV, and V) showed significant activity against breast, leukemia, and lung cell lines. These experimental data lead to the conclusion that oximes (II –V) may be regarded as potential starting structures for the design of novel anticancer agents. The results suggest that the II and III derivatives are suitable for in-vivo testing, according to the NCI (National Cancer Institute), since they have IC50 equal to or less than 4 µg /mL for all cell lines, whereas the compounds IV and Vin HL-60 cell line 26.
CONCLUSION: Oximes (II, III, IV, and V) showed significant activity against all cell lines. These experimental data lead to the conclusion that oximes (II –V) may be used as potential starting structures for the design of novel anticancer agents. These results are being published for the first time in this work.
ACKNOWLEDGEMENT: The authors wish to thank the Brazilian financial agencies CNPq.
CONFLICTS OF INTEREST: The authors declare that there are no conflicts of interest.
REFERENCES:
- World Health Organization: World's health ministers renew commitment to cancer prevention and control. http://www.who.int/cancer/media/news/cancer-prevention-resolution/en/ (11 July 2017,date last accessed).
- Rosas MSL, Silva BNM, Pinto RGMP, Silva BV, Silva AR, Guerra LR, Soares GCMT, Castro HC and Lione VOF: Incidência do Câncer no Brasil e o Potencial Uso dos Derivados de Isatinas na Cancerologia Experimental. Rev Virtual Quim 2013; 5(2): 243-65.
- Estimativa INCA: Incidência de câncer no Brasil." Rio de Janeiro: INCA (2016). http:// www. inca. gov. br / estimativa/2016/ (11 July 2017, date last accessed).
- Da-Silva AP; Farina M, Franco JL, Dafre AL and Kassa KJ: Kuca Temporal effects of newly developed oximes (K027, K048) on malathion-induced acetylcholinesterase inhibition and lipid peroxidation in mouse prefrontal cortex. Neuro Toxicology 2008; 29(1): 184-89.
- Soares SFCX, Vieira AA, Delfino RT and Figueroa-Villar JD: NMR determination of Electrophorus electricus acetyl cholinesterase inhibition and reactivation by neutral oxime. Bioorg Med Chem 2013; 1: 5923-30.
- Soga S, Sharma SV, Shiotsu Y, Shimizu M, Tahara H, Yamaguchi K, Ikuina Y, Murakata C, Tamaoki Kurebayashi TJ, Schulte TW, Neckers LM and Akinaga S: Stereospecific antitumor activity of radicicoloxime derivatives. Cancer Chemother Pharmacol 2001; 48(6): 435-45.
- Eyer P, Szinicz L, Thiermann H, Worek F and Zilker T: Testing of antidotes for organophosphorus compounds: experimental procedures and clinical reality. Toxicology 2007; 233(1): 108-19.
- Moshiri M, Darchini-Maragheh E and Balali-Mood M: Advances in toxicology and medical treatment of chemical warfare nerve agents. DARU Journal of Pharmaceutical Sciences 2012; 20(1): 81.
- Sharma R, Gupta B, Acharya J, Kaushik MP and Ghosh KK: Interactions between xylene-linked carbamoyl bis-pyridinium mono-oximes and organophosphates inhibited-AChE: a kinetic study. Toxicology 2014; 316: 1-8.
- Reuter S, Gupta SC, Chaturvedi MM and Aggarwal BB: Oxidative stress, inflammation and cancer: how are they linked. Free Radic Biol Med 2010; 49(11): 1603-16.
- Lima TC, Santos SRL, Uliana MP, Santos RLC, Santos, Brocksom TLSC, de Holanda Cavalcanti and de Sousa DP: Oxime derivatives with larvicidal activity against Aedesae gypti L. Parasitol Res 2015; 114(8): 2883-91.
- De Sousa DP, Schefer RR, Brocksom U and Brocksom TJ: Synthesis and Antidepressant Evaluation of Three para-Benzoquinone Mono-oximes and Their Oxy Derivatives Molecules 2006; 11(2): 148-55.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR: New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J Natl Cancer Inst 1990; 82(13) 1107-12.
- Junior VGM, Goncalves TDO, Regasini, Regasini LO, Ferreira PMP, Pessoa CDO, Costa Lotufo LV and Cavalheiro AJ: Cytotoxic clerodanedi terpenoids from Casearia obliqua. J Nat Prod 2009; 72(10): 1847-850.
- Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol Methods 1983; 65: 55-63.
- Sülsen VP, Cazorla SI, Frank FM, Redko FC, Anesini CA, Coussio DJ, Malchiodi EL, Martino VS and Muschietti LV: Trypanocidal and Leishmanicidal Activities of Flavonoids from Argentine Medicinal Plants. Am J Trop Med Hyg 2007; 77(4): 654-59.
- Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJL: Minor Cell viability assays- Assay Guidance Manual 2016; 1-36.
- International standard: Biological Evaluation of Medical Devices Part5: Tests for Cytotoxicity: in-vitro methods. ISO 1992; 10993-5.
- Rogero SO, Lugão AB and Ikeda TIAS: Cruz Teste in-vitro de Citotoxicidade: Estudo Comparativo entre duas Metodologias Mat Res 2003; 6(3): 317-320.
- Pessoa C, Silveira ER, Lemos TLG, Wetmore LA, Moreaes MO and Leyva A: Antiproliferative effects of compounds derived from plants of Northeast Brazil Phytother Res 2000; 14(3): 187-91.
- Bezerra DP, Pessoa C, Moraes MO, Alencar NM, Mesquita RO, Lima MW, Alves AP, Pessoa OD, Chaves JH, Silveira ER and Costa-Lotufo LV: In-vivo growth inhibition of sarcoma 180 bypiperlonguminine, an alkaloid amide from the Piper species. J Appl Toxicol 2008; 28(5): 599-07.
- Yang YC, Lim MY and Lee HS: Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal activity against three mosquito species. J Agric Food Chem 2003; 51(26): 7629-31.
- Cheng SS, Huang CG, Chen WJ and Kuo YHST: Chang Larvicidal activity of tectoquinone isolated from red heartwood-type Cryptomeria japonica against tewo mosquito species. Bioreso Technol 2008; 99(9): 3617-22.
- Huang S, Li R, Connolly PJ, Xu G, Gaul MD, Emanuel SL, Kenneth R, LaMontagne KR and Greenberger LM: Synthesis and biological study of 4-aminopyrimidine-5-carboxaldehyde oximes as antiproliferative VEGFR-2 inhibitors. Bioorg Med Chem Lett 2006; 16(23): 6063-66.
- [Martinez MWR, Militão GCG, Silva TG, Silva RO and Menezes PH: Synthesis of novel [3, 1]-benzothiazepine and [3,1]-benzoxazepinederivates with antitumoral activity. RSC Adv 2014; 4(28): 14715-147184.
- Brondani JD, Leite ACL, Nascimento SC, Lima RMOC and Bieber LW: Síntese, avaliação da atividade citotoxica e toxicidade aguda de análogos da Primina 3 e 6 Alquil-substituídas. Acta farm Bonaer 2006; 25(2): 248-51.
- Barros CJB, Souza ZC, Freitas JJR, Silva PBN, Militão GCG, Silva TG, Freitas JCR and Filho JRF: A conveniente synthesis and cytotoxic activity of 3-Aryl-5-pentyl-1,2,4-oxadiazoles from carboxylic acid esters and arylamidoximes under solent-free conditions. J Chil Chemi Soc 2014; 59(1): 2359-62.
How to cite this article:
Feitosa CM, Silva DSS, Militão GGC, Sousa DDP, Rashed K and Lima LKF: Benzoquinone mono oximes derivatives with anticancer activity. Int J Pharmacognosy 2020; 7(12): 369-75. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(12).369-75.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
5
369-375
596
938
English
IJP
C. M. Feitosa *, S. d. S. Silva, G. G. C. Militão, D. P. d. Sousa, K. Rashed and L. K. F. Lima
Post-graduate Programs in Chemistry and Pharmaceutical Sciences, Federal University of Piaui, Ininga, Teresina, Piauí, Brazil.
chistiane@ufpi.edu.br
24 August 2020
23 September 2020
22 November 2020
10.13040/IJPSR.0975-8232.IJP.7(12).369-75
31 December 2020