ASSESSMENT OF ANTI-INFLAMMATORY AND NEUROPHARMACOLOGICAL ACTIVITY OF GENDARUSSA VULGARIS LEAVES EXTRACT IN MICE
HTML Full TextASSESSMENT OF ANTI-INFLAMMATORY AND NEUROPHARMACOLOGICAL ACTIVITY OF GENDARUSSA VULGARIS LEAVES EXTRACT IN MICE
S. M. Mushiur Rahman *, Sharmin Naher, Koushik Ahammed, Trina Mony, Susmita Mistry Jui and Md. Abdullah Abu Sayeed
Department of Pharmacy, Faculty of Biological Science and Technology, Jessore University of Science and Technology, Jessore - 7408, Bangladesh.
ABSTRACT: In folk medicine, Gendarussa vulgaris Nees (Acanthaceae) is used for the treatment of pain, fever, asthma, rheumatism, colic’s, arthritis, jaundice, inflammation, cephalgia, eczema, diarrhea, wounds, dyspepsia. The present study was designed to evaluate for its safety as well as anti-inflammatory and neuro-pharmacological activities of ethyl acetate extract of Gendarussa vulgaris leaves (EAGVL) by using OECD guidelines, xylene-induced ear edema in mice, pentobarbital-induced sleeping test, open field, and hole cross test. Mortality, a sign of any toxicity or behavioral changes were not observed up to the dose as high as 4000 mg/kg. The crude extract was found to have significant (P<0.05, vs. control) anti-inflammatory activity at the oral dose of 200 mg/kg and 400 mg/kg (b.wt.) in the tested animals. Moreover, the extract of Gendarussa vulgaris leaves potentiated the pentobarbital-induced sleeping time in mice at dose 200 mg/kg and 400 mg/kg. In open field test, the extract at dose 200 mg/kg and 400 mg/kg showed a significant (P<0.05, vs. control) depressant and slight anti-depressant activity. Again, both lower and higher doses of extract (200 mg/kg and 400 mg/kg) of G. vulgaris leaves were decreased the number of passage through the hole from one chamber to other in hole cross test. The results obtained in the present study demonstrated that EAGVL can be the possible sources of anti-inflammatory, CNS depressant, anti-depressant and anxiolytic agents. But further investigation is needed for the identification of the active compounds as well as confirmation of their activities.
| Keywords: |
Gendarussa vulgaris, Acute toxicity, Anti-inflammatory, Neuropharmacological activity
INTRODUCTION: Plants provide complicated, mixed and distinct non-nutrient elements which act as the main basis of drug discovery 1. Gendarussa vulgaris (Family: Acanthaceae, commonly known as willow-leaved justicia, Nili nargandi, bakas, kala adulasa, kasanah, Gandharasa, vaidyasinha) is a small erect, fast-growing, branched shrub with attractive, lanceolate (shaped like a lance-head), ascending to spreading variegated leaves in shades of green, white and grey, and produces dainty white flowers.
It has been described as rare and endemic to India, though those claims are at least confusing, in the context of statements that the plant is widely used in various forms for many of its medicinal and insecticidal properties 2 and that it is a quick-growing, evergreen forest shrub considered to be a native of China and distributed in Bangladesh, Sri Lanka, India and Malaysia 3.
The plant is shrubby, about 2-4 ft. high. Leaves are simple, entire, opposite, lanceolate, variegated in shades of white, green and grey, 7 to 14 cm long and 1 to 2.5 cm wide, glabrous on both sides, apex acute-acuminate. The rather small flowers are borne in 4-12 cm long spikes, at the end of branches or in leaf axils. The teeth of the sepals cup are smooth, linear, and about 3 mm long. The flowers are about 1.5 cm long, white or pink, with purple spots. The capsule is club-shaped, about 1.2 cm long, and smooth. Capsule 1-2 inch long, clavate, glabrous; seeds unknown 4. The chemical constituents of the leaves include O-disubstituted aromatic amines, 2-aminobenzyl alcohol and their respective O-methyl ethers, friedelin, lupeol and β-sitosterol 5 which are revealed from the present study. In locally, the plant is considered as an emetic, emmenagogue, febrifuge, diaphoretic and leaves are traditionally used in the treatment of respiratory disorders like cough, cold, bronchitis, throat infections, pulmonary infections, arthritis, jaundice, cephalgia, hemiplegia, eczema, and allergic disorders like bronchial asthma, etc. 6
So, the present study was designed to justify the anti-inflammatory and neuropharmacological activities of Gendarussa vulgaris leaves, and evaluate the traditional usage scientifically.
MATERIALS AND METHODS:
Collection and Identification of the Plant: For performed this study, green and freshness leaves of Gendarussa vulgaris plant was collected from Jessore University of Science & Technology, Jessore, Bangladesh, in January 2018. The collected leaves were identified and confirmed by National Herbarium, Bangladesh.
Extraction: For ethyl acetate extraction, 300 g of powdered leaves were taken. First, the leaves of Gendarussa vulgaris were separated from the plant and thoroughly washed with fresh water to remove all dirt and contaminants and dried in the shade at room temperature (25 ± 2 °C) for two weeks. The materials were ground into coarse powder, and cold extraction method was used to extract the active components. The ground leaves (300 g) were soaked in sufficient amount (approximately 2 L) of ethyl acetate for 14 days at room temperature with periodical shaking and stirring.
The whole mixture was primarily filtered through cotton and then through Whatman no. 1 filters. The solvent was evaporated with a rotary evaporator under reduced pressure at 40 °C temperature to yield semisolid crude extract. The percentage yield of the extract was 2.97% (w/w). The extract was then preserved in a refrigerator until further use.
Experimental Animals: Nineteen Swiss albino mice of either sex, aged 4-5 weeks, weighing about 20-30 g were collected to run the experiment of anti-inflammatory and neuropharmacological activity, from the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh. Before initiating the experiment, the animals were exposed to alternative 12:12 h light and dark cycle at an ambient temperature of 26 ± 2ºC. Proper supplies of foods and water ad libitum were ensured.
All protocols for the animal experiment were approved by the Institutional Animal Ethical Committee of Jessore University of Science & Technology, Jessore, Bangladesh. Before run all experiment, mice were acclimatized for 7 days in the laboratory environment and maintained the constant environmental and adequate nutritional conditions throughout the experiment.
Acute Oral Toxicity Study: Acute toxicity has adverse effects that result either from a single exposure or from multiple exposures over a short time (normally less than 24 h). According to the Organization of Economic Cooperation and Development (OECD) guidelines, the acute toxicity study of Gendarussa vulgaris leaves was designed to estimate the half lethal dose (LD50) of the experimental samples 7. Ten mice were divided into two groups: control group and test group (EAGVL), with five animals per group. The experimental sample (EAGVL) was administered orally at different concentrations (100, 250, 500, 1000, 2000, 3000, and 4000 mg/kg body weight).
After that the animals were observed every 1 h for next 5-6 h for mortality, behavioral pattern changes such as salivation, weakness, aggressiveness, food or water refusal, diarrhea, discharge from eyes and ears, noisy breathing, changes in locomotor activity, convulsion, coma, injury, pain or any sign of toxicity in each group of animals. A final evaluation at the end of a 2-week observation period was also conducted 7.
Anti-inflammatory Study:
Xylene-Induced Ear Edema Test: Dai et al., 8 methods were used to evaluate xylene-induced ear edema in mice. Twenty mice were divided into four groups, as described before. Negative control (10mL/kg) received one dose of distilled water, where the standard group (100 mg/kg) treated with diclofenac sodium (DS) as well as test groups received EAGVL at 200 and 400 mg/kg orally. After one hour of the particular treatment, each animal received 20 µl of xylene on the anterior and posterior surfaces of the right ear lobe, where the left ear was kept untreated and considered as control. Mice were sacrificed by cutting off both ears with the utilization of 5 mm circular sections of the ears after 1 h of xylene application, then seized and finally weighed. The weight of xylene-induced edema was calculated from the difference between weight of ear treated with xylene (right ear) and the weight of ear left untreated (left ear).
The percentage inhibition of ear edema was calculated by the following formula:
Inhibition (%) = [1- Weight of edema (extract or standard drug) / Weight of edema (normal control)] × 100
Neuropharmacological Study:
Pentobarbital-Induced Hypnosis: The method of Williamson et al., 9 with slight modification was used for studying the pentobarbital-induced hypnosis test. Consisting of five mice in each group, the experimental animals were randomly divided into four groups. The groups were denoted from group-I to group- IV. Group I and II used as control and standard and group III and IV used as treatment groups. The experimental groups were administered with the ethyl acetate extract of Gendarussa vulgaris leaves at a dose of 200 mg/kg and 400 mg/kg body weight orally. Diazepam (1mg/kg p.o.) was administered as positive control, and negative control was treated with distilled water (10 mL/kg, p.o.).
Each mouse was placed in an observation box (a rectangular open box composed of hardboard floor (36 × 36 cm²) with a surrounding wall 30 cm height. After 30 min from the administration, pentobarbital (40 mg/kg, i.p.) was administered to each mouse to induce sleep. The total sleeping time was recorded for both controls as well as for treated groups. The animals were observed for the latent period (time between pentobarbitone administration to loss of righting reflex) and duration of sleep (time between the loss and recovery of righting reflex).
Open Field Test: In the case of the open field test, the method of Hawiset et al., 10 was applied. Grouping of the mice and sample (EAGVL at 200 and 400mg/kg body weight, p.o.) administration were carried out as like as pentobarbital-induced hypnosis test. The evaluation of the CNS depression activity can be completed by this test. An apparatus that consists of a series of alternating white and black squares floor with a height of 40 cm was made for the open field test. The number of movement of the test animals i.e., the total number of squares that every group of animals visited was counted at 0, 30, 60, 120 and 180 min after respective treatment and every counting was continued for 3 min.
Hole Cross Test: For the hole cross test, a slight modification of Takagi et al., 11 methods was followed. Twenty mice were divided into control group (distilled water, 10 mL/kg, p.o.), positive control or standard group (Diazepam, 1mg/kg, p.o.) and test groups (EAGVL at 200 and 400 mg/kg body weight, p.o.), containing five mice in each group. Here, a wood partition was fixed in the middle of a cage having a size of 30 × 20 × 14 cm. A hole of 3 cm diameter was made at the height of 7.5 cm in the center of the cage. The number of passages of each mouse through the hole from one chamber to other was counted for 3 min at 0, 30, 60, 120 and 180 min respectively after the oral administration of the test drugs and the standard. The apparatus was thoroughly cleaned after each trial.
Statistical Analysis: All experimental results were expressed as mean ± SEM (Standard Error of Mean). Statistical analyses for anti-inflammatory and neuropharmacological studies were evaluated by one-way ANOVA following Dunnett’s test through the SPSS software (version 16; IBM Corporation, New York, USA). The obtained results were compared with the vehicle control group. The P<0.05 was considered to be statistically significant.
RESULTS:
Acute Oral Toxicity Study: In acute oral toxicity study, no mortality was viewed up to the dose as high as 4000 mg/kg for EAGVL or control group. Any signs of toxicity or behavioral changes were not observed up to the dose as high as 4000 mg/kg for EAGVL (test group) or control group, before or after their administration in any animal, which lived up to 14 days. This indicated that the test group does not show acute oral toxicity.
Anti-inflammatory Study:
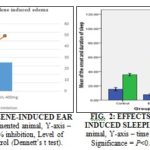
Xylene-induced Ear Edema Test: In Table 1, the result of the anti-inflammatory activity of EAGVL on topical xylene-induced ear edema in mice is shown. The cutaneous inflammation of mouse was rapidly obtained after xylene induced. All of the groups showed significant (P<0.05 vs. control) inhibition of ear edema and differences of ear weight. Percentage (%) of inhibition of both doses are 28.36% and 49.25% respectively, with 0.022 and 0.001 significance value. Among the extracts, 49.25% is the highest value of inhibition that was observed by EAGVL 400 mg/kg. These effects are illustrated in Fig. 1.
Neuropharmacological Study:
Pentobarbital-Induced Hypnosis: Statistical analysis of the data obtained in this test shows that Table 2 both 200 mg/kg and 400 mg/kg dose of ethyl acetate extract of leaves of Gendarussa vulgaris prolong the duration of the pentobarbitone-induced sleeping time.
TABLE 1: EFFECTS OF EAGVL ON XYLENE-INDUCED EAR EDEMA TEST
| Treatment Group | Dose mg/kg | Ear weight difference (mg) | Inhibition (%) | Significance |
| Control (vehicles) | 10 mL/kg | 13.40 ± 0.93 | - | - |
| Diclofenac sodium | 100 mg/kg | 6.20 ± 0.80 | 53.73 | .000 |
| EAGVL | 200 mg/kg | 9.60 ± 1.02 | 28.36 | .022 |
| EAGVL | 400 mg/kg | 6.80 ± 1.06 | 49.25 | .001 |
Ear weight differences are denoted as the mean ± standard error of the mean. P< 0.05 vs. control (Dunnett’s t-test).
TABLE 2: EFFECTS OF EAGVL ON PENTOBARBITAL-INDUCED SLEEPING TEST
| Group | Dose | Time of onset of sleep (min) | Total sleeping time (min) |
| Control | 10 mL/kg | 15.36 ± 1.13 | 35.60 ± 0.93 |
| Standard | 1 mg/kg | 4.73 ± 0.54 | 95.77 ± 2.54 |
| EAGVL | 200 mg/kg | 8.42 ± 1.16 | 58.76 ± 3.29 |
| EAGVL | 400 mg/kg | 6.13 ± 0.82 | 72.12 ± 3.62 |
Sleeping time and duration values are presented as mean ± SEM (standard error of the mean). P<0.05, vs. control (Dunnett’s t-test).
After completed this experiment, it was noted that the total sleeping time was about 58.76 ± 3.29 and 72.12 ± 3.62 min at a dose of 200 and 400 mg/kg of body weight, respectively of the methanolic leaves extract of G. vulgaris whereas, in positive control group sleeping time was about 95.77 ± 2.54 min. The effects are displayed in Table 2 and illustrated in Fig. 2.
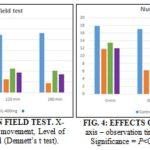
Open Field Test: It was observed that the extract (200 mg/kg and 400 mg/kg) of Gendarussa vulgaris leaves was elicited anti-depressive activity in first observation period in the test animals and then till last period (180 min) it elicited depressive activity. In this case, significant activities were noticed during all of the observations at EAGVL 400 mg/kg (P<0.05, vs. control).
Furthermore, slightly less result was obtained by EAGVL 200 mg/kg than EAGVL 400 mg/kg, and extract shows both depressive and anti-depressive activities, and all activities are dose-dependent in manner. Results of the open field test are showed in Table 3 and illustrated in Fig. 3.
Hole Cross Test: Results of hole cross test are showed in Table 4. The observation was similar as like as the open field test. Both doses of the extract were exhibited anti-depressive activity from the first observation period in the test animals, and then till last period (180 min), it elicited depressive activity. Also here, significant activities were noticed during all of the observations at EAGVL 400 mg/kg (P<0.05, vs. control). Furthermore, slightly less result was obtained by EAGVL 200 mg/kg than EAGVL 400 mg/kg, and extract shows both depressive and anti-depressive activities, and all activities are dose-dependent in manner. Results are illustrated in Fig. 4.
TABLE 3: EFFECTS OF EAGVL ON OPEN FIELD TEST
| No. of movement in an opened field | ||||||
| Group | Dose | 0 min | 30 min | 60 min | 120 min | 180 min |
| Control | 10 mL/kg | 117.30 ± 5.98 | 114.47 ± 8.26 | 111.91 ± 2.91 | 108.58 ± 5.50 | 111.87 ± 4.50 |
| Standard | 1 mg/kg | 102.22 ± 2.48 | 55.07 ± 2.64 | 39.25 ± 2.24 | 25.01 ± 2.18 | 17.72 ± 2.33 |
| EAGVL | 200 mg/kg | 108.10 ± 3.79 | 75.59 ± 3.23 | 62.46 ± 3.48 | 44.60 ± 3.43 | 40.75 ± 3.69 |
| EAGVL | 400 mg/kg | 104.43 ± 3.67 | 68.45 ± 4.28 | 54.40 ± 2.86 | 37.54 ± 3.13 | 33.84 ± 3.29 |
Numbers of movement in the open field are present as mean ± SEM (standard error of the mean). P<0.05, vs. control (Dennett’s t-test)
TABLE 4: EFFECTS OF EAGVL ON HOLE CROSS TEST
| No. of movements | ||||||
| Group | Dose | 0 min | 30 min | 60 min | 120 min | 180 min |
| Control | 10 mL/kg | 17.80 ± 1.71 | 17.00 ± 1.14 | 18.40 ± 2.20 | 16.60 ± 1.60 | 16.40 ± 1.81 |
| Standard | 1 mg/kg | 11.80 ± 1.36 | 6.20 ± 1.28 | 2.60 ± 0.40 | 1.60 ± 0.51 | 1.60 ± 0.51 |
| EAGVL | 200 mg/kg | 13.40 ± 1.23 | 9.30 ± 1.51 | 8.12 ± 1.65 | 6.90 ± 1.29 | 5.62 ± 1.18 |
| EAGVL | 400 mg/kg | 12.01 ± 1.09 | 8.38 ± 1.27 | 7.49 ± 1.34 | 4.38 ± 1.06 | 3.90 ± 0.81 |
Numbers of movement are present as mean ± SEM (standard error of the mean). P<0.05, vs. control (Dennett’s t-test)
DISCUSSION: Scientifically rigorous toxicity studies have been conducted on very few, although many plant-derived products are in use in systems of traditional medicine. It is mandatory to know about acute oral toxicity studies for the assessment of the exact range of doses for subsequent usage as well as recognition of the significant adverse effects of the materials under examination.
The acute oral toxicity study is a vital factor for the investigation of the therapeutic index of drugs and xenobiotics 12. As no mortality was observed up to the dose as high as 4000 mg/kg, LD50 of Gendarussa vulgaris leaves extract could not be obtained. For this, the extract was found to be safe with a broad therapeutic range, and two comparatively high doses (200 mg/kg and 400 mg/kg) of EAGVL were used for in-vivo doses.
Inflammation is a part of the complex biological response of vascular tissues to harmful stimuli such as pathogens, damaged cells, or irritants. Inflammation has four cellular processes, which are changes in blood flow by changing in smooth muscle cell function that is accountable for vasodilatation, alteration of the vascular permeability, migration phagocytic leukocytes to the site of inflammation, and phagocytosis 13. Xylene-induced ear edema test is done as an acute inflammatory test. Also, xylene can release inflammatory mediators such as bradykinin, histamine, and serotonin. These mediators are responsible for edema as they enhance vascular permeability and improve vasodilation 14. Fluid accumulation occurs at the treatment site, which is shown by the xylene-induced ear edema test and inhibition of this fluid accumulation is considered as anti-inflammatory effect 15.
Diclofenac sodium is a cyclooxygenase inhibitor. It inhibits pros-taglandin synthesis and somewhat cycloxygenase-2 selective. The ethyl acetate extract has an activity which is comparable to diclofenac sodium can be said to inhibit the cyclooxygenase enzyme but lipoxygenase inhibitors also possess significant anti-inflammatory activity. Here, the leaves of Gendarussa vulgaris contains β-sitosterol, phenolic and flavonoids compounds which produced significant inhibition of ear edema that may be due to the blockage of phospholipase A2, reduction of vascular permeability, and vasodilation and reduce inflammation 16. But the extensive study is required to assure the exact mechanism, through which the extracts suppressed edema. The assessment of CNS activity of any drug depends on the locomotor activities of animals. The estimation of the level of excitability of the CNS refers to the locomotor activity of the animal. An increase in alertness is regarded as locomotor activity, and reduction in locomotor activity is an indication of sedative effect 17.
Neuropharmacological disorder such as- depression and anxiety both are important psychiatric imbalance that badly affects a person’s quality of life and social relations directly. The results of depression and anxiety in the community are very high and are associated with a lot of morbidities. These are characterized by emotional symptoms such as loss of self- confidence, hopelessness, apathy, sense of guilt, indecisiveness, and a motivation, as well as biological symptoms like sleep disturbances, psychomotor retardation, loss of libido, and loss of appetite. The major depression is considered when symptoms are very severe. In spite of, the availability of several drugs in the market, it is very important to address these problems and find effective remedies. Cause, all chemically synthetic drugs are associated with some limitations, and there is an urgent need for alternative medications for these disorders. Medical therapies with medicinal herbs may be more effective alternatives in the treatment of depression and anxiety. And the research of their effects has progressed significantly since the past decade 18, 19.
This study examined some neuropharmacological effects of Gendarussa vulgaris leaves and established that it has antidepressant and anxiolytic activities. EAGVL increased the pentobarbitone-induced sedative effect in mice and it is a dose-dependent manner. Between two doses EAGVL 400mg show more significant depressant activity than EAGVL 200 mg/kg. To generate their action, barbiturates naturally work on the cerebral cortex 20. EAGVL improved sleeping time that can be attributed to its action on the central sleeping mechanism. Moreover, EAGVL decreased the locomotor activity which is a parameter of the level of excitability of the central nervous system. Decrease of locomotor activity is closely related to the depression of the central nervous system 21.
Flavonoids and phenolic compounds have been reported to have multiple biological effects such as Central nervous system disorders 22. Also, the study on locomotor activity, as measured by hole cross and open field tests, showed that both doses (200 mg/kg and 400 mg/kg) of methanol extract from the leaves of Gendarussa vulgaris gradually decreased the frequency and the number of movements with time. The sedative effect recorded here may be related to an interaction with benzodiazepines and related compounds that bind to receptors in the CNS and have already been identified in certain plant extracts. Many flavonoids and neuroactive steroids, and especially the β-amyrin acetate were found to be ligands for the GABAA receptors in the CNS; which led to the hypothesis that they act as benzodiazepine-like molecules 23. This is supported by the present study on the behavioral effects in animal models of anxiety and sedation.
Probably, the mechanism of anxiolytic action of the ethyl acetate extract of Gendarussa vulgaris leaves could be due to the binding of any of the phytoconstituents to the GABAA-BZD complex. In support of this, it has been found that flavones bind with high-affinity BZD site of the GABAA receptor 24. By the locomotor activity, it is possible to measure the level of excitability of the CNS and sedation resulting from depression of the central nervous system 25. So, we can conclude that the present study seems to support the claims of a traditional medicine practitioner about the use of Gendarussa vulgaris in inflammation and CNS disorder.
CONCLUSION: From the existing study, it could suggest that Ethyl acetate extract of Gendarussa vulgaris leaves might possess remarkable anti-inflammatory and neuropharmacological activities. Data obtained in this study showed that all activities were dose-dependent and statistically significant. The presence of flavonoids, alkaloids, sitosterol, tannin, and phenolic compounds might be responsible for these activities and which are probably mediated via inhibition of various autocoids formation and release.
We hope that a further detailed investigation is underway to determine the exact phytoconstituents that are responsible for these activities. The genotoxicity study of this extract may be a promising area for the researchers. Moreover, it could be a potential source for novel ‘lead’ discovery for anti-inflammatory and neuro-pharmacological drug development.
ACKNOWLEDGEMENT: For providing facilities throughout the work, the authors would like to thank the Department of Pharmacy, Jessore University of Science & Technology, Jessore, Bangladesh.
CONFLICT OF INTEREST: The authors declare that there is no conflict of interests regarding the publication of this manuscript.
REFERENCES:
- Ali SK, Hamed AR, Soltan MM, El-Halawany AM, Hegazy UM and Hussein AA: Kinetics and molecular docking of vasicine from Adhatoda vasica: an acetylcholinesterase inhibitor for Alzheimer’s disease. South African Journal of Botany 2016; 104: 118-124.
- Agastian P, Lincy Williams and S Ignacimuthu: In-vitro propagation of Justicia gendarussa Burm. f. - A medicinal plant. Indian Journal of Biotechnology 2006; 5: 246-248.
- Thomas TD and Yoichiro H: In-vitro propagation for the conservation of a rare medicinal plant Justicia gendarussa Burm. f. by nodal explants and shoot regeneration from callus. Acta Physiol Plant 2010; 32: 943-950.
- Burkill IH and Clarke CB: Flora of Tropi Africa 1900; 1.
- Arokiyaraj S, Perinbam K, Agastian P and Balaraju K: Immunosuppressive effects of medicinal plants of Kolli Hills on the mitogen-stimulated proliferation of human peripheral blood mononuclear cell in-vitro. Indian J Pharmacol 2007; 39: 180-183.
- Warrier PK, Nambiar VPK and Ramankuttey C: Indian medicinal plants -A compendium of 500 species. Orient Longman Limited, Chennai 1995; 3: 272-273.
- Walum E: Acute oral toxicity. Environ Health Perspect 1998; 106 (S-2): 497-503.
- Dai Y, Liu LH and Kou JP: Anti-inflammatory effect of aqueous extract of Wu-HU-Tang,” China Pharmaceutical University 1995; 6: 362-364. https://www.ncbi.nlm.nih. gov/pubmed/2600603.
- Williamson EM, Okpako DT and Evans FJ: Pharmacological methods in phytotherapy research, John Willey & Sons, England, Edition 1st, 1996: 184.
- Hawiset T, Muchimapura S, Wattanathorn J and Sripanidkulchai B: Screening neuropharmacological activities of Kaempferia parviflora (Krachai dam) in healthy adult male rats. American Journal of Applied Sciences 2011; 8: 695-702.
- Takagi K, Watanabe M and Saito H: Studies on the spontaneous movement of animals by the hole cross test: Effect of 2-dimethylaminoethane. Its acylates on the central nervous system. Japan Journal of Pharmacology 1971; 21: 797.
- Sahni C, Shakil NA, Jha V and Gupta RK: Screening of nutritional, phytochemical, antioxidant and antibacterial activity of the roots of Borassus flabellifer (Asian Palmyra Palm). Journal of Pharmacognosy and Phytochemistry 2014; 3(4): 58-68.
- Okokon JE and Nwafor P: Anti-inflammatory, analgesic and antipyretic activities of ethanol root extract of Croton zambesicus. Pak J Pharm Sci 2010; 23(4): 385-392.
- Barbosa-Filho JM, Piuvezam MR and Moura MD: Anti-inflammatory activity of alkaloids: a twenty-century review, Revista Brasileira de Farmacognosia 2006; 16(1): 109-139.
- Sokeng SD, Koube J and Dongmo JF: Acute and chronic anti-inflammatory effects of the aqueous extract of Acacia nilotica (L.) Del. (Fabaceae) pods. Academia Journal of Medicinal Plants 2013; 1(1): 1-5.
- Shripad BM, Abhijeet AA, Inayat PB and Nitin N: Analgesic and anti-inflammatory evaluation of Ficus microcarpa L. leaves extract; Asian J Pharm Clin Res 2012; 5(S-4): 258-261.
- Verma A: Pharmacological Evaluation of Saraca indica leaves for central nervous system depressant activity in mice. J of Pharma Sci and Res 2010; 2: 338-343.
- Hasrat JA, De Bruyne T, De Backer JP, Vauquelin G and Vlietinck AJ: Isoquinoline derivatives isolated from the fruit of Annona muricata as 5-HTergic 5-HT1A receptor agonists in rats: Unexploited antidepressive (lead) products. J Pharm Pharmacol 1997; 49(11): 1145-9.
- Hasrat JA, Pieters L, De Backer JP, Vauquelin G and Vlietinck AJ: Screening of medicinal plants from Suriname for 5-HT (1A) ligands: Bioactive isoquinoline alkaloids from the fruit of Annona muricata. Phytomedicine 1997; 4(2): 133-40.
- Bowman WC and Rand MJ: Textbook of Pharmacology, Black-well Scientific, New York, NY, USA, 1980.
- Ozturk Y, Aydin S, Beis R, Baser HKC and Berberoglu H: Effects of Hypericum perforatum Linn. and Hypericum calycinum Linn. extracts on the central nervous system in mice, Phytomedicine 1996; 3(2): 139-146.
- Priyanka B, Sridhar Y and Shankaraiah P: Antidepressant and muscle relaxant activity of Cardiospermum halicacabum Linn. Roots in mice. Journal Advanced Pharmaceutical Sciences 2012; 2(1): 193-200.
- Jäger AK and Saaby L: Flavonoids and the CNS. Molecules 2011; 16: 1471-1485. Johnston GAL GABA (A) receptor channel pharmacology. Curr Pharm Des 2005; 11: 1867-1885.
- Hanrahan JR, Chebib M and Johnston GAR: Flavonoid modulation of GABAA receptors. Br J Pharmacol 2011; 163(2): 234-245.
- Thirupathy KP, Tulshkar A and Vijaya C: Neuro-pharmacological activity of Lippia nodiflora Linn. Pharmacognosy Res 2011; 3(3): 194- 200.
How to cite this article:
Rahman SMM, Naher S, Ahammed K, Mony T, Jui SM and Sayeed MAA: Assessment of anti-inflammatory and neuropharmacological activity of Gendarussa vulgaris leaves extract in mice. Int J Pharmacognosy 2018; 5(11): 738-45. doi link: http://dx.doi.org/10.13040/ IJPSR.0975-8232.IJP.5(11).738-45.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
7
738-745
708
1221
English
IJP
S. M. M. Rahman *, S. Naher, K. Ahammed, T. Mony, S. M. Jui and M. A. A. Sayeed
Department of Pharmacy, Faculty of Biological Science and Technology, Jessore University of Science and Technology, Jessore, Bangladesh.
smushiurjustphar@gmail.com
26 September 2018
21 October 2018
23 October 2018
10.13040/IJPSR.0975-8232.IJP.5(11).738-45
01 November 2018