APPLICATIONS OF SURFACTANTS IN PHARMACEUTICAL FORMULATION DEVELOPMENT OF CONVENTIONAL AND ADVANCED DELIVERY SYSTEMS
HTML Full TextAPPLICATIONS OF SURFACTANTS IN PHARMACEUTICAL FORMULATION DEVELOPMENT OF CONVENTIONAL AND ADVANCED DELIVERY SYSTEMS
Urvashi Khare *, P. K. Sharma and Amrish Kumar
Institute of Pharmacy, School of Medical and Allied Sciences, Galgotias University, Greater Noida - 201310, Uttar Pradesh, India.
ABSTRACT: The role of surfactants is very critical in the development of pharmaceutical formulations. The diverse applications in different delivery systems have been highlighted in different reports of formulation development for various conventional and advanced delivery systems. The relevancy of critical micelle concentration and HLB value for the section of surfactants for different drug delivery systems was rationalized with suitable examples. The present attempt is focused on the compilation of all the important aspects of surfactants with a broad discussion on the drug delivery implications. The regulatory status of different investigational surfactants has been criticized relevantly with a compilation of FDA approved surfactants. The safety aspects of newly developed surfactants must be ensured before their incorporation in therapeutic dosage forms. The properties of different categories of surfactants were discussed with special emphasis on their physicochemical properties. The compiled information will make a firm base for the selection of surfactants for conventional and advanced drug delivery systems. Precise information on surfactant toxicity was also included to give a check to the safe concentrations of these surface active agents in pharmaceutical formulation meant for administration through different routes of administration. The various pharmaceutical applications of surfactants were also discussed in detail.
| Keywords: |
Critical micelle concentration, HLB Value, Phase behavior of surfactants, Surfactant toxicity
INTRODUCTION: When two phases which are immiscible with each other comes in contact, then the boundary between them is known as the interface. There are several types of interface depending on the types of adjacent phases, such as solid, liquid, or gaseous state. The term surface is commonly used when the interface is formed between either a gas-solid or a gas-liquid interface. Every interface acts as a surface.
In the pharmaceutical formulation development, one has to deal with the different interfacial phenomenon which affects the characteristic properties of the dosage form or delivery system under consideration. To deal with such interfacial phenomenon in the pharmaceutical dosage form, surfactants are incorporated in the formulations 1. Surfactants are surface active agents which are amphiphilic. These are interfacially active compounds having both polar (Hydrophilic) and non-polar (Lipophilic) feature within the same molecule 2.
The interfacial phenomenon has a significant effect in the field of pharmacy and medicines as it plays a key role in the drug absorption onto solid adjuncts in dosage form, molecules penetration through the biological membrane, and the dispersion of immiscible materials to form emulsion 3. Surfactants are also helpful to enhance the solubility of insoluble therapeutic moiety due to their amphiphilic nature; they possess both oil soluble and water soluble characteristics. Surfactants have also shown the capability to be used as drug carriers. As non-ionic surfactants are widely used to form stable solutions of drugs which are poorly soluble in water. Apart from non-ionic surfactants phospholipids are also used as a tool for drug delivery. To develop a delivery system containing surfactants, the potential and limitations of such systems can be understood by the phase behavior of the surfactants.
Critical Micelle Concentration: In solutions surface, active agent form micelles or aggregates and the process of micelles formation is known as Micellization, or in other words, Micellization is a strongly cooperative self-association process occurring at particular narrow concentration, critical micellar concentration.
The hydrophobic effect is believed to be the main driving force in the self association. Surfactant reduces the free energy in the system by decreasing the hydrophobic surface area. Furthermore, micelles act as the reservoir for the surfactant molecule as a single monomer unit 4.
TABLE 1: CHARACTERISTIC VALUES OF CMC AND NUMBER OF SURFACTANT MOLECULES PER MICELLE FOR SOME OF THE IMPORTANT SURFACTANTS 4
| Name
|
Type of
surfactant |
CMC
(mmol/m3) |
No. of surfactant molecules per micelle |
| Potassium laurate | Anionic | 2.4 × 104 | 50 |
| Sodium octane sulfonate | Anionic | 15×104 | 28 |
| Sodium decane sulfonate | Anionic | 4×104 | 40 |
| Sodium dodecyl sulfate | Anionic | 0.8×104 | 62 |
| Decytrimethyl ammonium bromide | Cationic | 6.3×104 | 36 |
| Tetradecyltrimethyl ammonium chloride | Cationic | 0.3×104 | 64 |
| Dodecylammonium chloride | Cationic | 1.3×104 | 55 |
| Polyoxyl 8 dodecyl ether | Non-ionic | 0.013×104 | 132 |
| Polyoxyl 12 dodecyl ether | Non-ionic | 0.014×104 | 78 |
| Nonoxynol 10 | Non-ionic | 0.07×104 | 276 |
| Nonoxynol 30 | Non-ionic | 0.024×104 | 44 |
The capability of the surfactant molecule to lower the interfacial surface tension depends on the free monomer concentration at which formation of micelles starts is known as critical micelles concentration. Micelles are spherical or cylindrical. Ionic surfactant has higher CMC value than the non-ionic because of the electrostatic repulsion of the head group makes micellization more difficult. A representative of CMC values and aggregation numbers of surfactant molecules per micelles are listed in Table 1. 5
Phase Behaviour of Surfactant: 6 Route of administration of for insertion of surfactant in the body is important, which is better understood by the phase behavior of surfactants. If surfactants are diluted below its CMC, it may lead to precipitation of drug which is administered. Such kind of precipitation is most commonly seen in the case of intravenous administration. Non-ionic surfactants are preferred over ionic for being used in the delivery system as they possess lower CMC.
A) Equilibrium Phase Structure: As surfactants self-associate in a wide variety of solvents, hence their aggregation varies between solvents. As the continuous phase in most of the delivery systems are water or buffer solutions, it is important to predict the range of equilibrium phase structure that is incorporated in such solutions. Surfactant generally aggregate when dispersed in water above its CMC, aggregation depends on the molecular geometry into any of the four types isotropic micellar phase, liquid crystalline, hexagonal lamellar and cubic phase. Apart from these some of the usual structures are also observed such as helical bi-layers and fiber gels.
B) Modified Phase Structure: Apart from the equilibrium phase, there are some non-equilibrium and modified structures also used in drug delivery. Vesicles are formed by lamellar phase dispersion in excessive water or non-aqueous phase. Reverse vesicles are formed by dispersion over oil. These non-equilibrium structures are prepared using methods such as sonication and will eventually re-equilibrate back into the lamellar phases from which they originate. Polymerized aggregates are made to use polymerization to stabilize various developing phase structures. Micelles polymerization has the significant in-vivo advantage that these structures do not breakdown on dilution. Because of their size (ranging from tens to hundreds of nm), these aggregates can cause problems as they are not readily excreted from the body, hence such systems will probably have limited clinical use, although they may have a use in oral administration. Biodegradable polymerized aggregates are presently being investigated
Hydrophilic-Lipophilic Balance Value: In 1947, Griffith developed a system called hydrophilic-lipophilic balance (HLB) for selecting the surfactants according to their Hydrophilic/ lipophilic nature. Griffith explained HLB value for a surfactant as the mol% of the hydrophilic group divided by 5. HLB value indicates the polarity of the surfactants. It is also used to describe the functional properties of the surfactants. Concerning formulation under development surfactants having high polarity are assigned higher value while the surfactants with low polarity are assigned lower value. The ionic nature of the surfactant is an important consideration for its incorporation in any formulation 4. The function and properties of surfactants can be represented in terms of HLB values Table 2.
TABLE 2: PROPERTIES OF SURFACTANTS AND THEIR HLB VALUE
| Properties | HLB value |
| Oil soluble | <10 |
| Water soluble | >10 |
| Antifoaming | 4–8 |
| Water in oil emulsifier | 7–11 |
| Oil in water emulsifier | 12–16 |
| Wetting agent | 11–14 |
| Detergent | 12–15 |
| Stabilizer | 16–20 |
Lipophilic surfactants used to couple water-soluble material into non-aqueous oil-based system whereas hydrophilic surfactant is used for solubilization and detergency. The HLB value of surfactants depends on oil type and the temperature of the solution 7. Identifying the correct HLB number can be done by observing an indication when the small quantity of surfactant is mixed with water and shaken. The appearance of the solution indicates the HLB number. The more the hydrophilic surfactant higher is the HLB number 8.
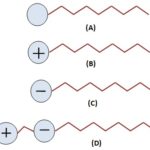
Classification of Surfactants: Surfactants are classified into four groups depending upon the charge possessed by their polar group as cationic, anionic, amphoteric, and non-ionic. Fig. 1 represents the types of surfactants. Table 3 represents a hydrophilic group of surfactants. The different active drugs for therapeutic delivery possess different natures, i.e. as the hydrophilic part of surfactant may have positive, negative, or both charges or may have no charges.
FIG. 1: TYPES OF SURFACTANT (A) NON-IONIC (B) CATIONIC (C) ANIONIC (D) AMPHOTERIC
A) Anionic Surfactants: These surfactants possess negative charge on their head group while the straight tail chains are composed of saturated/unsaturated C12-C18 aliphatic group. The potential for aqueous solubility of these surfactants depends on the double bonds present in the molecule. Owing to their superior hair conditioning property, anionic surfactants are preferred for the formulation of shampoo’s meant for oil removal from hairs. Deactivation of anionic surfactants is observed when mixed with water due to the higher concentration of cations (Ca++ and Mg++) present in water 9. Anionic surfactant can be classified based on functional groups at their head, g. sulfonate, phosphate, sulfate, and carboxylate 10.
B) Cationic Surfactants: These surfactants possess a positive charge on their head. Cationic surfactants are having single long alkyl group exhibit good aqueous solubility while surfactants with multiple long alkyl groups (hydrophobe) are dispensable in water and exhibit solubility in organic solvents 11.
TABLE 3 TYPICAL HYDROPHILIC GROUP OF SURFACTANTS 11, 33, 34
| Type | Examples | Hydrophilic Group | Structure |
| Anionic | Sodium lauryl sulfate, alkyl benzene, sodium laureth sulfate, diglyceride amino phosphoric acid, propane 1,2,3-triol, sodium dilaurate-7 citrate | Sulfate | -OSO2O- |
| Sulphonate | -SO2O- | ||
| Ether sulfate | -(OCH2CH2)nOSO2O- | ||
| Ether phosphate | -(CH2CH2O)nP(O)O- | ||
| Ether carboxylate | -(CH2CH2O)nCO2- | ||
| Carboxylate | -C(O)O-- | ||
| Cationic | Cetyl trimethyl ammonium bromide, benzalkonium chloride, cetyl pyridinium chloride | Primary ammonium | -N+ H3- |
| Secondary ammonium | -N+(R)H2- | ||
| Tertiary ammonium | -N+(R)2H- | ||
| Quaternary ammonium | -N+(R)3- | ||
| Amphoteric | Lecithins, betaines, alkyl amino propionic acid, sodium coco glycinate | Amine oxide | -N+(R)3O-- |
| Betain | -N+H(R)2(CH2)nC(O)O-- | ||
| Aminocarboxylate | -N+H(R)3(CH2)n C(O)O-- | ||
| Non-ionic | Cetyl alcohol, stearyl alcohol, alkyl polyglucosides, tweens, spans | Polyoxyethylene | -(OCH2CH2)nOH- |
| Acetylenic | -CH(OH)CCH(OH)- | ||
| Monoethanolamine | -NHCH2CH2OH- | ||
| Diethanolamine | -N(CH2CH2OH)2 |
C) Amphoteric Surfactants: The head of these surfactants is composed of both positive and negative charge groups. They are also known as zwitterionic surfactants. These are soluble in water but have minimal solubility at isoelectric point. They are stable at both alkaline and acidic pH of the solution in which they are dissolved 12. These surfactants acquire positive charge in acidic pH solution and show characteristics similar to cationic surfactants while at alkaline pH they acquire negative charge in the alkaline pH and shows characteristic of the anionic surfactant. It can be explained as:
The surface activity of the amphoteric surfactants depends on the distance between the charged groups. Hence, shows maximum surface activity at isoelectric point. The most commonly used class of amphoteric surfactant is N-alkyl betaine which is derived from trimethylglycine, e.g. lauryl amido propyl dimethyl betaine. Another classes are N-alkyl amino propionates and alkyl imidazoline. Zwitterionic surfactants are best used in dermatological products. They have low eye and skin irritability, hence used in shampoos and cosmetics. They show good antistatic property and are foam boosters 13.
D) Non-Ionic Surfactant: These do not produce ions in aqueous solutions. Hence, they are compatible and excellent candidates to enter complex mixtures. These are less sensitive to electrolytes, particularly divalent cations than ionic surfactant can be used with high salinity. These are good emulsifier and wetting agents and have good foaming properties. They exhibit low toxicity level. The most commonly used non-ionic surfactants are ethers of fatty alcohols. The sorbitan esters are water-insoluble whereas soluble in alcohol and have low HLB value 14.
Surfactant Protein: Total 10% of the protein by weight has been isolated from the surfactant. Major parts of these proteins are having serum protein, i.e. about 80% and the remaining 20% are specific to surfactant.
TABLE 4 TYPES OF SURFACTANT PROTEINS, THEIR MOLECULAR CHARACTERISTIC, AND FUNCTIONS 16
| Type | Molecular Characteristic | Main
Function |
| SP-A | Lectin/collagen hybrid | Supports alveolar macrophage activities and regulate surfactant secretion |
| SP-B | Disulfide bridge | Optimize surface activity |
| SP-C | Rich in hydrophobic valine | Optimize surface activity |
| SP-D | Lectin/collagen hybrid | Interacts with alveolar macrophage and regulate surfactant secretion |
Till date, there are four surfactant specific proteins identified are termed as surfactant protein A, B, C and D according to the decreasing order of their molecular weight. Surfactant Protein A is most commonly used and found in the alveolar space 15. Table 4 represents the types of surfactant proteins.
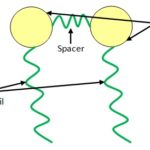
Gemini Surfactant: These are dimeric molecules consisting of two head groups linked together with a short spacer and two hydrophobic tails. They have a high affinity in lowering the surface tension and very low CMC. This effect is due to the good packing of the gemini surfactant molecules at the air/water interface. Use of Gemini surfactant shows special interest as the drug vesicles and in gene therapy. GS can be synthesized at low cost, hence are advantageous for pharmaceutical industries. Cationic-serine based GS have improved the interfacial properties and lowered toxicity showing potential use in the biological application. GS has more ability than other surfactants in dispersing CNTs. Nimoipine-loaded egg phosphatidylcholine-sodium glycocholate mixed micelles improves the water solubility of nimidipine, thus enhancing their chemical use 17. Fig. 2 represents the structure of gemini surfactant. Srivastava et al. observed the synergetic effect of the mixed system due to hydrophobic interaction between Gemini surfactant and tetracaine hydrochloride, which were used to stabilize the silver nanoparticles 18.
FIG. 2: STRUCTURE OF GEMINI SURFACTANT
Pharmaceutical Application of Surfactants:
A) As Permeation/Absorption Enhancers: Ionic surfactants are enhanced transdermal absorption by disordering the lipid bilayer of the stratum corneum and by denaturation of keratin. Azone (1-dodecylazacycloheptan-2-one or laurocapram) is one of the most efficient enhancers of percutaneous absorption. It enhances the penetration of hydrophilic and lipophilic compounds. Azone leads to the fluidization of the intercellular lipid lamellar part of the stratum corneum 19. Same as with DMSO it reduces the resistance of the lipid barrier and thus increases the lipid fluidity. Alcohol derivatives of N, N disubstituted amine acids, and hexamethylene lauramine also enhance the permeability of the drug 20.
B) In Respiratory Distress Therapy: Surfactant preparations are used in the treatment of neonatal respiratory distress syndrome (also known as hyaline membrane disease) in premature infants. These preparations are used in combination with supplement oxygen and mechanical ventilation to facilitate a gas exchange for either prophylactic or rescue treatment of neonatal respiratory syndrome 21.
C) As Flocculating Agent: These are used to decrease the rate of sedimentation of floccules example of such agents are tragacanth, carbopol 934, methylcellulose, vegum or bentonite used either alone or in combination. Addition of an anionic electrolyte example monobasic potassium phosphate is used for flocculating positively charged particles 21.
D) In Suppository Bases: Several non-ionic surfactants are used in the development of suppository bases. Some of these bases are used in formulating both oil and water-soluble drugs 22. Polyoxyethylene sorbitan fatty acids esters (tween), polyoxyethylene stearates, and the sorbitan fatty acid esters (span) are some surface active agents used in the formulation of suppositories 23.
E) In Suspension Aerosols: These reduce agglomeration thus leads to an increase in the stability of the suspension. These orient at the solid-liquid interface and coat each particle in the suspension aerosols are non-ionic surfactants and those surfactant whose HLB value is less than 10. 24
F) For Contact Lens Cleaning: Surfactants emulsify accumulated oils, inorganic compounds and lipid over the contact lenses, hence are good cleansers. They are used by just placing the drop of surfactant over the lens and gently cleaning the lens back and forth or used with mechanical washing device. Non-ionic detergents, wetting agents and buffers are used as ingredients for cleaning 25, 21.
G) As Emulsifying Agent: Lipophilic part of the surfactants is generally responsible for the surface activity. Depending on the individual nature of the surfactant these are used to form o/w and w/o emulsions 26. Non-ionic surfactants are effective at the pH range of 3-10, the cationic surfactant is effective over pH range of 3-7, here as anionic surfactants are effective at the pH range more than 8. 13, 27
H) As Cerumen Removing Solutions: The waxy deposition in the external auditory canal, which is composed of secretions of sweat and sebaceous gland, is known as cerumen. Some of the synthetic surfactants have been utilized for their cerumenolytic activity to remove the ear wax, g. triethanolamine polypeptide oleate-condensate (Cerumenex drops) combination with propylene glycol. Another example is carbamide peroxide in combination with glycerine/propylene glycol (Debrox drops). Carbamide peroxide, when comes in contact with oxygen, destroys the impacted wax, which leads to the easy removal of wax 28.
I) In the Petroleum Industry: Surfactants show great diversity and practical importance in the petroleum industry and are advantageous in the production of petroleum processes like in the reservoir of oil and gas wells, in surface processing operations. CMC is of great importance in the selection of the surfactant to be used in the petroleum industry. For example enhance recovery of oil process includes the use of surfactant involving micellar, alkali/ surfactant/ polymer (A/S/P) and gas (hydrocarbons, N2, CO2 or steam) flooding. The surfactant must be present in the concentration more than CMC because the effect of surfactant is obtained at the significant CMC whether it is lowering of interfacial tension or for promoting the stability 29, 30.
Regulatory Status for Surfactants: Any substance which is added to the food is recognized as additives, which are the subject of approval by the Food and Drug Administration (FDA). Generally Recognised as Safe (GRAS) under sections 201(s) and 409 of the federal food, drug, and cosmetic act. Under section 201(s) and 409 and FDA’s implementing regulations in 21 CFR(Code of Federal Regulations) 170.3 and 21 CFR 170.3, the additive must be GRAS either through scientific procedures or for an additive used in food before 1958. Table 5 shows some FDA approved surfactants 10.
TABLE 5: FDA APPROVED SURFACTANTS WITH THEIR COLOUR, MELTING POINT AND HLB VALUE 10
| Name | Colour | Melting point | HLB value | FDA status |
| Sorbitan monostearate | Ivory colored | 128 – 132 °F | 4.7 | Approved |
| Polyoxyethylene (20) sorbitan monostearate | Yellow oily fluid | 600 cp | 14.9 | Approved |
| Polysorbate 80 | Yellow oily fluid | 400 cp | 15.0 | Approved |
| Polyoxyethylene (20) sorbitan Tristearate | Tan waxy solvent | ~ 92 °F | 10.5 | Approved |
| Mono and diglycerides | Ivory white colored solid | 135 – 142 °F | 3 | GRAS |
Surfactant Toxicity: The most common type of toxicity caused by surfactants is in the aspects of dermatology. Surfactants can get adsorbed on the surface of the biomembrane due to the hydrophobic interactions with proteins. It binds on the interface and leads to the membrane disruption, which affects the normal functioning of the cell. Interaction of surfactant with red blood cells (RBC) can lead to hemolysis 31.
Anionic surfactants are more toxic as compared to the non-ionic surfactants example sodium dodecyl sulfate (SDS) used in toothpaste is more irritant than ether sulfate used in hand dishwashing formulations. Some of the amphoteric surfactants such as betaines are also used to reduce the skin irritation caused by anionic surfactant 32.
It has also been reported that the surfactants have shown a negative effect on micro-organisms example phosphate solubilizing Acinetobacter junii, autotrophic ammonia oxidizing Nitrosomonas. SDS and Triton X 100 (non-ionic surfactants) affect the nitrogen-fixing ability and growth of cyanobacteria Gloecapsa at a concentration of 50 and 500 ppm. LAS has the capability to destabilize protease and amylase activity of bacteria Bacillus licheniformis at a lower concentration than CMC 11.
Patents on Surfactants: Holtze et al., developed surfactant system containing hydrophilic tail used to stabilize aqueous or hydrocarbon droplets in fluoronic continuous phase. The developed system was composed of a fluorophilic tail and a head group so as to create a surfactant with a suitable geometry to stabilize the reverse emulsion containing aqueous or lipophilic droplets in a fluorophilic continuous phase. Policello et al. worked on trisiloxane compounds to improve the delivery of active ingredients as aqueous solutions. When used in aqueous solutions, trisiloxane type compounds have been reported to enable the control over wetting, spreading, foaming, and detergency processes to achieve the desired effect. However, these compounds were reported to be effective only in a narrow pH range, i.e. from a slightly acidic pH of 6 to a very mildly basic pH of 7.5. Table 6 provides the details about the patents on surfactants.
TABLE 6: PATENTS ON SURFACTANTS
| Patent no. | Year | Title | Inventors | Reference |
| US 9,012,390 B2 | 2015 | Fluorocarbon emulsion stabilizing Surfactants | Holtze et al., | 35 |
| 9018150 | 2015 | Cleansing composition with cationic surfactants | Kirolos Rizk | 36 |
| US 8,197.841 B2 | 2012 | Polymerizable surfactants and comonomers | Linhardt et al., | 37 |
| US 8,235,120 B2 | 2012 | Mesophase fluids with extended Chain surfactants for downhole treatments | Quintero et al. | 38 |
| US 7,935,842 B2 | 2011 | Hydrolysis resistant Organomodified trsiloxane Surfactants | Policello et al., | 39 |
Surfactant-Drug Interaction: Akram M. analyzed that cationic gemini surfactants show stronger binding constant with drug ibuprofen (IBF) than the other surfactants Kb values obtained through the techniques: Intrinsic fluorescence, UV-Visible, and CV follow the trend as C12-E2O-C12 > C14-E2O-C14 > C16-E2O-C16 because of higher penetration efficiency of ibuprofen in the looser micelles. Intrinsic fluorescence also illustrates that the hydrogen bonding and van der Waal forces play a dominant role in the exothermic binding of IBF- Cm-E2O-Cm. Surfactants are used to increase the rate of dissolution of some drugs 40.
Pan Fang showed that surfactants possess the property of membrane lysis in bacteria. Most of the studied surfactant showed membrane-lytic property above their CMC, but some like C12TAB and anionic SDS showed the membrane-lytic property below their CMC, causing structural damage to the membrane, mitochondria, and nuclei of bacteria. While with the increase in acyl chain length of SME surfactants showed a sharp decline in membrane lysis and with the physical feature of concentration-dependent also showed cytotoxicity and bactericidal action against E. coli and S. aureus 41.
Smedt and Raemdonk discussed that pulmonary surfactants could also be used for surfactant replacement therapy for the treatment of respiratory distress syndrome. Surfactant proteins can be used in drug delivery as they modulate the pulmonary distribution of drugs for the treatment of lung cancer and pulmonary lung disease. Accordingly, Wu and Colleagues investigated the conjugation of an anti-SP-C antibody to liposome for dual purpose, i.e. increasing the retention time and targeting microRNA delivery to alveolar type II cells. The intranasal administration of LP-ODNanti-SP-C showed high in-vivo specificity for type II cells, with retention time up to 48 h 42. Tozuka prepared alternate formulation of DCP SDC and DCP-Stevia-G and analyzed the improved property of micellization when compared to single components. Negative values of interaction parameters and free energy showed that interaction between SDC/stevia-G and DCP forms stable mixed micelles. Also showed increase adsorption of nanosized micelles and mixed micelles the cell membrane and delivered dug to the target cells. Hence, formulation consisting of nanosized drug and surfactant showed reduced cytotoxicity and improved the solubility of poorly soluble water-soluble drugs 43.
Azum N. Studied the interaction of antipsychotic drug chlorpromazine (CPL) hydrochloride with mixed surfactant (CTAB and 16-4-16), which are used to reduce the side effects of CPZ. Gemini surfactants are used instead of conventional as they have lower CMC values, which tends to reduce the carrying amount and also increase the amount of drug to be incorporated. The formation of mixed micelle with negative values of free energy showed that the adsorption process is primary, whereas the process of Micellization is secondary 44.
CONCLUSION: For the development of novel and conventional dosage form, the role of surfactants is well established. However, their rational use according to the different route of administration is to be closely monitored to avoid toxic effects. Furthermore, extensive safety studies are required to establish the safety profile of newer surfactant at the investigational stage.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: The authors declare no conflict of Interest.
REFERENCES:
- Mishra M, Muthuprasanna P, Prabha KS, Rani PS, Babu IAS, Chandiran IS and Shalini S: Basics and potential applications of surfactants - A review. International Journal of Pharm Tech Research 2009; 1(4): 1354-65.
- Sekhon BS: Surfactants: Pharmaceutical and medicinal aspects. Journal of Pharmaceutical Technology, Research and Management 2013; 1(1): 43-68.
- Jhon C: Anionic surfactant: Analytical chemistry. Merckel Dekker series, Edition 2nd, 1998.
- Felton LA: Remington: Essential of pharmaceutics. Pharmaceutical Press, Edition 22th, 2013.
- Logan JW and Moya FR: Animal-derived surfactant for the treatment and prevention of neonatal respiratory syndrome: Summary of clinical trials. Ther Clin Risk Manag 2009; 5(1): 251-60.
- Lawrence Jayne M: Surfactant systems: Their use in drug delivery. Chemical Society Reviews 1994; 417-24.
- surfactants.com/HLBsystem.html 07-05-99
- Zhang W, Dai X, Zhao Y, Lu X and Gao P: Comparison of different type of surfactant for effect on activity and structure of soybean peroxidize. Langmuir 2009; 25(4): 2363-8.
- Wong O, Huntington J, Nishihata T and Rytting JH: New alkyl N, N-dialkyl substituted amino acetates as transdermal penetration enhancers. Pharm Res 1989; 6(4): 286-95.
- Azarmi R and Ashjaran A: Type and application of some common surfactants. Journal of Chemical and Pharmaceutical Research 2015; 7(2): 632-40.
- Attwood D and Florence AT: Surfactant system their Chemistry Pharmacy and Biology. Chapman and Hall, Edition 1st, 1983.
- Corrigan OI and Healy AM: Surfactants in pharmaceutical products and systems, In: Encyclopedia of Pharmaceutical Technology, Taylor and Francis, Edition 3rd, Chapter 258, 2006: 3583-96.
- Sekhon BS: Surfactants: Pharmaceutical and medicinal aspects. Journal of Pharmaceutical Technology, Research and Management 2013; 1(1): 43-68.
- Kothencz R, Nagy R, and Bartha L: Determination of HLB values of some non-ionic surfactants and their mixtures. Studia Universitatis Babeș-Bolyai Chemia 2017; 62(4): 451-58.
- Persson B, Chang D, Rust K, Moxley M, Longmore W and Crouch E: Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry 1989; 28: 6361-67.
- Hamm H, Kroegel C and Hohlfeld J: Surfactant: A review of its functions and relevance in adult respiratory disorders. Respiratory Medicine 1996; 90(5): 251-70.
- Sekhon BS: Gemini (dimeric) surfactants. Resonance 2004; 42-49.
- Srivastava A, Thapa U, Saha M and Jalees M: Aggregation behaviour of tetracaine hydrochloride with Gemini surfactants and the formation of silver nanoparticles using drug-Gemini surfactants mixture. Journal of Molecular Liquids 2018; 276: 399-08.
- Lava R: Overview of synthetic detergents, 2017; 27-28.
- ZP L, Liu Q and Lu XW: Effect of Azone of different concentration on percutaneous absorption of baicalin in-vitro. Di Yi Jun Yi Da Xue Xue Bao 22(11): 1003-4.
- Mishra M, Muthuprasanna P, Prabha KS, Rani PS, Babu IAS, Chandiran IS and Shalini S: Basics and potential applications of surfactants - A review. International Journal of Pharm Tech Research 2009; 1(4): 1354-1365.
- Falbe J: Surfactants in Consumers Products 1987.
- Realdon N, Zotto D, Morpurg M and Franceschinis M: Effect of surfactant characteristics on drug availability from suppositories. E Pharmazie 2008; 63(6): 459-63.
- Li X, Gu L, Xu Y and Wang Y: Preparation of fenofibrate nanosuspension and its Pharmacokinetics behaviour rates in rats. Drug Dev Ind Pharm 2009; 35(7): 827-33.
- Giménez-Arnau A, Gilaberte M, Conde D, Espona M and Pujol RM: Contact Dermatitis 2007; 57(1): 61.
- Rebello S, Asok AK, Mundayoor S and Jisha MS: Pollutant Diseases. Remediation and Recycling 2013; 4.
- Salaguer JL: Surfactants types and uses. Laboratory of Formulation, Interfaces. Rheology and Processes 2002; 2: 1-49.
- Dimmitt P and Pediatr J: Cerumen removal products. Health Care 2005; 19(5): 332-6.
- Surfactants C and Ammonium Q. (n.d.). Surfactant List Surfactant List (cont) Non - ionic Surfactants 866.
- The Dow Chemical Company. A Broad Range of Anionic and Non-ionic Products. Dow Surfactants Reference Chart. 2014.
- Ivankovic T, Hrenovic J and Gudelj I: Toxicity of commercial surfactants to phosphate accumulating bacterium. Acta Chim Slov 2009; 56.
- Rebello S, Asok AK, Mundayoor S and Jisha MS: Pollutant Diseases. Remediation and Recycling 2013; 4.
- Brandt KK, Hesselso M, Roslev P, Henriksen K and So J: Toxic effects of linear alkylbenzene sulfonate on metabolic activity, growth rate, and microcolony formation of Nitrosomonas and Nitrosospira strains. Appl Environ Microbiol 2001; 67: 2489-98.
- https://www.lion-specialty-chem.com.co.jp/en/product/structure/s03.htm
- Holtze: Fluorocarbon emulsion stabilizing Surfactants. 2015. US 9,012,390 B2.
- Rizk K: Cleansing composition with cationic surfactants. 2015. 9018150.
- Linhardt: Polymerizable surfactants and comonomers. 2012. US 8,197.841 B2.
- Quintero: Mesophase fluids with extended Chain surfactants for downhole Treatments. 2012. US 8,235,120 B2.
- Policello: Hydrolysis resistant Organomodified trsiloxane Surfactants. 2011. US 7,935,842 B2.
- Akram M, Anwar S, Bhat IM and Kabir-ud-din: Exploration of ibuprofen binding with micellar assemblies of the efficiently-engineered gemini surfactant: Insight from spectroscopic and volumetric studies. Colloids and Surface A: Physicochem Eng Aspects 2018.
- Pan F, Li Z, Gong H, petkov JI and Lu JR: Membrane-lytic actions of sulphonated methyl ester surfactants and implications to bactericidal effect and cytotoxicity, Journal of Colloidal and Interface Science 2018; 531: 18-27.
- Guagliardo R, Perz-gil J, Smedt SD and Koen R: Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. Journal of Controlled Release 2018.
- Srivastava A, Uchiyama H, Wada Y, Hatanaka Y, Shirakava Y, Kadota K and Tozuka Y: Mixed micelles of the anti-histaminiccationic drug diphenhydramine hydrochloride with anionic and non-ionic surfactants show improved solubility, drug release and cytotoxicity of ethenzamide. Journal of Molecular liquids 2018.
- Azum N, Naqui AZ, Rub MA, and Asiri AM: Multi-technique approach towards amphiphilic drug-surfactant interaction: A physiochemical study. Journal of Molecular Liquids 2018.
How to cite this article:
Khare U, Sharma PK and Kumar A: Applications of surfactants in pharmaceutical formulation development of conventional and advanced delivery systems. Int J Pharmacognosy 2019; 6(5): 155-63. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.6(5).155-63.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
155-163
637
1407
English
IJP
U. Khare *, P. K. Sharma and A. Kumar
Institute of Pharmacy, School of Medical and Allied Sciences, Galgotias University, Greater Noida, Uttar Pradesh, India.
urvashi13051994@gmail.com
13 April 2019
23 May 2019
25 May 2019
10.13040/IJPSR.0975-8232.IJP.6(5).155-63
31 May 2019