ANTIOXIDANT, ANTI-INFLAMMATORY AND ANTIMICROBIAL ACTIVITY OF WHOLE PLANT OF TECOMA CAPENSIS THUMB.
HTML Full TextANTIOXIDANT, ANTI-INFLAMMATORY AND ANTIMICROBIAL ACTIVITY OF WHOLE PLANT OF TECOMA CAPENSIS THUMB.
Kota Padmaja * 1, 2, Ganapaty Seru 2, Jagadeesh Panda 1 and Mary Sulakshna 1
Raghu College of Pharmacy 1, Dakamarri, Bheemunipatnam (M), Visakhapatnam - 531162, Andhra Pradesh, India.
GITAM Institute of Pharmacy 2, Gandhinagar, Rushikonda, Visakhapatnam - 530045, Andhra Pradesh, India.
ABSTRACT: Objective: The study was designed to explore the anti-oxidant, anti-microbial and anti-inflammatory activity of Tecoma capensis Thumb. (T. capensis) whole plant extract. Material and Method: Antioxidant and free radical scavenging activity of extract of ethanolic extract of Tecoma capensis was investigated against 2, 2 Diphenyl-1-picrylhydrazyl (DPPH) assay, superoxide radical and hydroxyl radical scavenging activity compared with that of standard (ascorbic acid) and antimicrobial activity was evaluated against clinically important bacterial strain those were Bacillus subtilis, Staphylococcus aureus (gram +ve), Escherichia coli, Proteus vulgaris (gram -ve) and two fungal strain Aspergillus niger, Candida albicans using cup plate method. Anti-inflammatory activity was evaluated by carrageenan-induced rat paw edema method by the oral administration of EETC (100, 250 mg/kg). The data were statistically analyzed by ANOVA. Results: The IC50 values were determined by radical scavenging activity of EETC by DPPH (21 ± 0.06), superoxide radical (37.2 ± 0.04) and hydroxyl radical scavenging activity (12 ± 1.64). The average zone of inhibition for EETC against S. aureus (15 ± 0.816) and E. coli (15.2 ± 0.5). Anti-inflammatory activity was evaluated by carrageenan-induced rat paw edema method by the oral administration of EETC (100, 250 mg/kg) exhibited significant and dose-dependent anti-inflammatory activity. Conclusion: it is concluded that the EETC showed significant and dose-dependent activities. Therefore, this plant can be used for new pharmacological research accomplishment.
| Keywords: |
Antioxidant, Antimicrobial and Anti-inflammatory, EETC (Ethanolic extract of Tecoma capensis)
INTRODUCTION: Tecoma capensis (T. capensis) Thumb. (Bignoniaceae), commonly called a cape- honeysuckle, an evergreen and fast-growing scrambling shrub which may go up to 2-3 m high and spread more than 2.5 m. The plant loses its leaves in a colder climate.
It has pinnately compound leaves that have oval leaflets with blunt teeth. Flowers are orange in color, tubular and attracting nectar-feeding insects and birds. The bark infusion is used to treat sleeplessness to induce sleep 1 and also used as an analgesic, anti-inflammatory and antipyretic 2.
The leaves were used to treat pneumonia and intestinal trouble 3. The activities reported were antiulcer 4, anti-microbial 5 anti-pyretic 6, anti-inflammatory 7, 8, hepatoprotective 9 and cytotoxic 10. Phyto-constituents reported were different iridoid glucosides from the leaves 11. In this article author wants to explain the detailed antioxidant activity by three methods, antimicrobial activity against two gram +ve, two gram -ve and two fungal strains and anti-inflammatory activity by carrageenan-induced rat paw edema method which is not yet published.
Plant Material: The whole plant of Tecoma capensis Thumb (Bignoniaceae) was collected from Chandana Mohan Rao Nursery Gardens, Visakhapatnam and authenticated by the Botanical Survey of India (BSI/DRC/2015-16/Tech/684), Hyderabad, Telangana, India.
Preparation of Plant Extract: Whole plants were thoroughly washed with water. The collected plants were cut into small pieces and dried under shade. The dried pieces were ground into fine powder by using a mixer grinder and extracted by maceration with ethanol for 7 days with occasional shaking and stirring. Then, the liquid was concentrated under vacuum with a rotary vacuum to get dried extract.
Animals: Anti-inflammatory activity experiment was carried out using 20 Albino rats of weight 200-250g male / female of age 12 weeks. All the experimental procedure and protocols used in this study are reviewed and approved by the Institutional Animal Ethical Committee (IAEC) of the Raghu College of pharmacy contributed by the guidelines of the CPCSEA, government of India (1549/PO/Re/S/2011/ CPCSEA).
All the rats were given a period of acclimatization for 14 days before starting the experiments. Rats were fed a pellet diet, and water ad libitam, temperature maintaining at 24 ± 2 °C and relative humidity 60-70%. These rats were divided into four different groups, each containing five animals; the animals were marked individually.
Drugs and Chemicals: The chemicals used for these experiments were procured from various sources i.e. DPPH (CDH Pvt. Ltd. New Delhi), Ascorbic acid (Yarrow chem Mumbai), NBT (CDH Pvt. Ltd. New Delhi), riboflavin (CDH Pvt. Ltd. New Delhi), EDTA (CDH Pvt. Ltd. New Delhi), methanol (Fisher scientific Mumbai), sodium dihydrogen phosphate (Yarrow Chem Mumbai), di-sodium hydrogen phosphate (Yarrow Chem Mumbai), de-oxy ribose (CDH Pvt. Ltd. New Delhi), potassium dihydrogen phosphate (Yarrow Chem Mumbai), ferric chloride (Fisher Scientific Mumbai), hydrogen peroxide (Yarrow chem Mumbai), thiobarbituric acid (CDH Pvt. Ltd. New Delhi), trichloroacetic acid (Fisher Scientific Mumbai), carrageenan (Sigma), indomethacin (Recon, Bangalore).
Anti-oxidant Activity:
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay: 12 The free radical scavenging activity of ethanolic extract of Tecoma capensis was measured by DPPH (1, 1-diphenyl-2-picryl-hydrazil) employing the method described by Blois, 1958 with slight modification. 0.1 mM solution of DPPH in ethanol was prepared, and 1 ml of this solution was added to 3 ml of various concentrations (5, 10, 15, 20, 25, 30, 35, 40, 45, 50, μg/ml) of ethanolic extract. After 30 min, absorbance was measured at 517 nm. The percentage of inhibition was calculated by comparing the absorbance values of the control and test samples. Ascorbic acid was used as a reference compound. The capability to scavenge the DPPH radical was calculated using the following equation:
% DPPH scavenging activity = (Abs control – Abs test)/ Abs control ×100
Where, Abs control is the absorbance of the control reaction and Abs test is the absorbance in the presence of the sample of the extracts/standard. The antioxidant activity of the extract was expressed as IC50. The IC50 value was defined as the concentration (in μg/ml) of extracts that inhibits the formation of DPPH radicals by 50%.
Hydroxyl Radical Scavenging Activity: 13 Hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and EETC for hydroxyl radical generated by Fe3+-Ascorbate–EDTA-H2O2 system (Fenton reaction). The reaction mixture contained in a final volume of 1.0 ml, 100 μl of 28 mM 2-deoxy-2- ribose in 20 mM KH2PO4-KOH buffer of pH 7.4, 500 μl of the selected concentrations of extract (0.1, 0.5, 1, 5, 10, 20, 50, 100, 150, 200, 250, 300, 350, 450, 500, 700, 1000 μg/ml) in KH2PO4-KOH buffer (20 mM, pH 7.4), 100 μl of 1.04 mM EDTA, 100 ml 200 mM FeCl3, 100 μl of 1.0 mM H2O2 and 100 μl of 1.0 mM ascorbic acid was incubated at 37 °C for 1 h. 1.0 ml of thiobarbituric acid (1%) and 1.0 ml of trichloroacetic acid (2.8 %) were added to the test tubes and were incubated at 100 °C for 20 min. After cooling, absorbance was measured at 532 nm against control containing deoxyribose and buffer. Ascorbic acid was used as a positive control. Reactions were carried out in triplicate. The percentage inhibition was determined by comparing the results of the test and control compounds by using the following formula
Hydroxyl scavenging activity (%) = (Abs control – Abs test)/ Abs control × 100
Where, Abs control is the absorbance of the control reaction and Abs test is the absorbance in the presence of the sample of the extracts/standard.
Determination of Superoxide Radical Scavenging Activity: 14 Superoxide is biologically important as it can form singlet oxygen and hydroxyl radical. Overproduction of superoxide anion, radical contributes to redox imbalance and associated with harmful physiological consequences. Superoxide anion is generated in PMS-NADH system by the oxidation of NADH and assayed by the reduction of NBT resulting in the formation of blue formazan. 100 µl of Riboflavin solution [20 µg], 200 µl EDTA solution [12 mM], 200 µl methanol and 100 µl NBT (Nitro-blue tetrazolium) solution [0.1 mg] were mixed in a test tube, and the reaction mixture was diluted up to 3 ml with phosphate buffer [50 mM]. The absorbance of the solution was measured at 560 nm using phosphate buffer as blank after illumination for 5 min.
This is taken as control. 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, μg/ml of different concentrations of extract, as well as standard preparation, were taken and diluted up to 100 µl with methanol. To each of these, 100 µl Riboflavin, 200 µl EDTA, 200 µl methanol and 100 µl NBT was mixed in test tubes and further diluted up to 3 ml with phosphate buffer. Absorbance was measured after illumination for 5 min. at 560 nm on UV visible spectrometer Shimadzu, UV-1601, Japan. The superoxide anion scavenging activity was calculated according to the following equation.
% Inhibition= (Abs control – Abs test) / Abs control ×100
Where, Abs control is the absorbance of the control reaction and Abs test is the absorbance in the presence of the sample of the extracts/standard. The IC50 values for EETC, as well as standard preparation, were calculated 15, 16.
Calculation of 50 % Inhibition Concentration: The graph was plotted by taking concentration on X-axis and percentage inhibition on Y-axis, the graph was extrapolated to find the 50% inhibition concentration of the sample.
Antimicrobial Activity:
Test Microorganism & Inoculums Preparation: The test organisms used in this study were as follows: Bacillus subtilis, Staphylococcus aureus (gram +ve), Escherichia coli, Proteus vulgaris (gram-ve) and two fungal strain Aspergillus niger, Candida albicans for the antimicrobial tests, procured from MTCC (Microbial type culture collection), IMTECH (Institute of Microbial Technology), Chandigarh, (Punjab), maintained on nutrient agar slants (oxide) at 4 °C, a loop-full culture of all microorganism was inoculated into nutrient broth under sterile conditions, and incubated at 37 °C for 24 h in rotary shaker.
Antimicrobial Activity: 17 The agar disc diffusion method was used to evaluate the antibacterial potentiality of EETC against various gram +ve, gram -ve, and fungal strain. A 100 µl of freshly prepared inoculum was spread onto the surface of sterile Mueller Hinton agar using sterilized glass spreader. A sterile borer was used to prepare the cups of 8 mm diameter in the agar medium spread with micro-organism, and 0.1 ml of inoculums was spread on the agar plate by spread plate technique. Accurately measured (0.05m1) solution of each concentration and reference standard (ciprofloxacin 50 µg/ml and fluconazole 0.1 mg/ml) was added to the cups with a micropipette. All the plates were kept in a refrigerator at 2 °C to 8 °C for effective diffusion of test compounds and standards for 2 hours. Later, they were incubated at 37 °C for 24 hours. The presence of a definite zone of inhibition of any size around the cup indicated antibacterial activity. The solvent control (DMSO 4.0%, v/v) was run simultaneously, which was used as a vehicle. The diameter of the zone of inhibition was measured and recorded.
Anti-inflammatory Activity:
Acute Toxicity: The acute toxicity of Tecoma capensis was assessed by using up and down method 18. After the administration of one single dose of EETC (5, 50, 300, 2000 mg/kg) the survival of animals was observed during 24 h. If an animal survived at any given dose, the dose for the next animal was logarithmically increased; if it died, the dose was decreased.
Anti-inflammatory Activity: 19 Male or female Albino rats are starved overnight and grouped into five. To ensure uniform hydration, the rats receive pure drinking water. At the time of the experiment they were not allowed access to both feed and water. The rats are subjected to intraperitoneal injection of 0.05 ml of 1 % solution of carrageenan into the plantar side of the right hind paw.
The three groups of rats an hour post intraperitoneal (IP) administration were subjected to 100, 250 mg/kg of test extract and 20 mg of standard. The standard and test drug is dissolved or suspended in the same volume. The vernier calipers [RSK, Mumbai] was used to measure paw width and thickness before, and at 1st, 2nd, 3rd, 4th, 5th and 6th hour after an injection of carrageenan the paw volume was then calculated from the width (a) and thickness (b) measurement using the following Equation:
Volume = π×a2×b
The percentage inhibition of paw edema was calculated for each group concerning its vehicle-treated control group by using the formula:
Percentage inhibition of edema = (1−Vt /Vc) × 100
Statistical Analysis: The result were expressed as the mean ± SD (standard deviation) for three parallel measurements using graph pad prism version 6.0 for windows and subjected to statistical analysis by student t-test for comparison between groups. In all the cases P< 0.05 was considered as statistically significant. Data were computed for statistical analysis by using graph PAD prism.
RESULTS AND DISCUSSION:
Antioxidant Activity: In this study, the antioxidant activity of EETC was compared with ascorbic acid and shown in various in-vitro tests, i.e. DPPH scavenging activity, superoxide radical scavenging activity, and hydroxyl radical scavenging activity.
DPPH Scavenging Activity: The DPPH, stable radical has been widely used in the determination of primary antioxidant activity, i.e., the free radical activity of the pure antioxidant compound (standard) and the extract. The assay was based on the reduction of DPPH radicals in methanol, which causes decreasing absorbance at 517 nm.
TABLE 1: IC50 % VALUE OF IN-VITRO ANTIOXIDANT ACTIVITIES OF TECOMA CAPENSIS
| S.
no. |
sample | DPPH radical
scavenging activity |
Hydroxyl radical
scavenging activity |
Superoxide radical scavenging activity |
| 1 | Tecoma capensis | 21 ± 0.06 | 12 ± 1.64 | 37.2 ± 0.04 |
| 2 | Ascorbic acid | 12 ± 0.05 | 5 ± 1.18 | 23 ± 1.04 |
Data are presented as mean ± SD
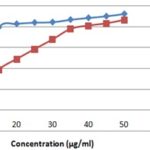
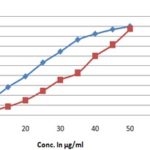
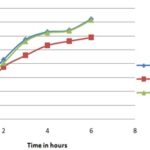
FIG. 1: DPPH RADICAL SCAVENGING ACTIVITY OF ETHANOLIC EXTRACT OF TECOMA CAPENSIS AND ASCORBIC ACID. Data are presented as the percentage of DPPH radical scavenging, Mean ± SD.
FIG. 2: DPPH RADICAL SCAVENGING ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS AND ASCORBIC ACID. Data are presented as the percentage of DPPH radical scavenging, Mean ± SD.
FIG. 3: DETERMINATION OF HYDROXYL RADICAL SCAVENGING ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS AND ASCORBIC ACID. Data are presented as the percentage of hydroxyl radical scavenging, Mean ± SD.
FIG. 4: DETERMINATION OF HYDROXYL RADICAL SCAVENGING ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS AND ASCORBIC ACID. Data are presented as the percentage of hydroxyl radical scavenging, Mean ± SD.
The scavenging effect increased with increasing with the increasing concentrations of the test compounds. The IC50 was calculated for the extract as well as standard and summarized in Table 1 and graphically represented in Fig. 1 and 2. The IC50 value of the extract by DPPH scavenging activity was 21 ± 0.06, and ascorbic acid was 12 ± 0.05, which was comparatively lower than the standard. It means ethanolic extract of Tecoma capensis at higher concentration captured more free radicals formed by DPPH resulting in a decrease in absorbance and the increase in IC50 value.
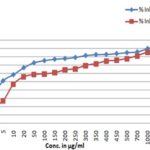
Hydroxyl Radical Scavenging Activity: The scavenging ability of EETC on hydrogen peroxide is shown in Fig. 3 and 4 and compared with ascorbic acid as standard. The EETC was capable of scavenging hydrogen peroxide in an amount dependent manner. At a concentration of 1 mg/ml, the scavenging activity of the extract and the standard was found to be 87.49 ± 0.34268 and 90.05 ± 0.1743 respectively. The IC50 value of the extract was (12 ± 1.64) was found to be more effective in quenching the hydroxyl radicals produced in the reaction mixture. The hydroxyl radical can induce oxidative damage to DNA, lipids, and proteins. Thus removing the H2O2 is very important for antioxidant defense in a cell by various food materials like fruits, green leafy vegetables, etc. The hydroxyl radical scavenging ability of extract was determined by its ability to compete with deoxyribose for hydroxyl radical. The extract diminishes the chromogen formation by competing with deoxyribose. In this assay, 2-deoxy-2-ribose was oxidized when exposed to hydroxyl radicals generated by Fenton-type reaction. The oxidative degradation can be detected by heating the product with TBA under acidic conditions to develop a pink chromogen (thiobarbituric acid reactive species) with a maximum absorbance at 532 nm 21.
Superoxide Radical Scavenging Activity: Superoxide radical is one of the strongest ROS among the free radical and gets converted to other harmful reactive oxygen species such as hydrogen peroxide and hydroxyl radical damaging bio-molecules which result in chronic diseases 22.
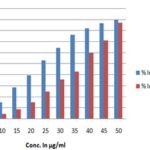
FIG. 5: DETERMINATION OF SUPEROXIDE RADICAL SCAVENGING ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS AND ASCORBIC ACID. Data are presented as the percentage of superoxide radical scavenging, Mean ± SD.
FIG. 6: DETERMINATION OF SUPEROXIDE RADICAL SCAVENGING ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS AND ASCORBIC ACID. Data are presented as the percentage of superoxide radical scavenging, Mean ± SD.
These radicals generated from dissolved oxygen by PMS-NADH coupling can be measured by their ability to reduce NBT. The decrease in absorbance at 560 nm with the plant extract (EETC) and the reference compound ascorbic acid indicates their abilities to quench superoxide radicals in the reaction mixture. The IC50 value of the extract is 37.02 ± 0.04, and the standard is 23 ± 1.04 respectively shown in Table 1 and Fig. 5 and 6.
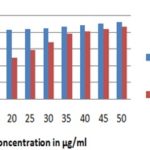
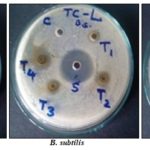
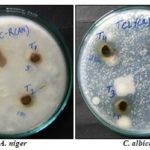
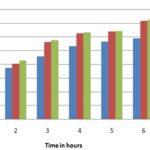
Antimicrobial Activity: The antimicrobial activities of the plant extract (EETC) against four bacterial strains and two fungal strains were examined and assessed by the presence or absence of inhibition zones. The inhibition zones of the extract tested for antibacterial and antifungal activity are given in Table 2, 3 and Fig. 7, 8 shows potential gram +ve and gram-ve activity but very less antifungal activity. S. aureus causes infections including superficial skin lesion, localized abscesses, and food poisoning 23. B. subtilis causes meningitis, endocarditis, pneumonia, wound infection and lesions 24. E. coli are Gram-negative bacteria causing a severe type of food poisoning and also causes a liver abscess, infra abdominal obstetric and gynecologic. Enteropathogenic strains of E. coli cause acute diarrhoea in children below 2 years and may cause acute death due to the dehydration and electrolyte imbalance 25, and P. vulgaris causes wound infection and urinary tract infection 26.
TABLE 2: ZONES OF GROWTH INHIBITION (mm) SHOWING ANTIBACTERIAL ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS; WELL DIAMETER 8.0 mm
| Name of the organism | Standard
(Ciprofloxacin 50 µg/ml) |
T1 (50 mg of EEAN) | T2 (100 mg of EEAN) | T3 (150 mg of EEAN) | T4 (200 mg of EEAN) | Control |
| S. aureus (Gram + ve) | 24.83 ±
0.2886 |
12.25 ±
0.5 |
14 ±
0.8165 |
14.5 ±
0.5774 |
15 ±
0.816 |
- |
| B. subtilis (Gram +ve) | 24.66 ±
0.5773 |
10.5 ±
0.5773 |
12.5 ±
0.5773 |
12.75±
0.5 |
14.5 ±
0.5773 |
- |
| P. vulgaris (Gram -ve) | 24.33 ±
0.5773 |
12.5 ±
0.5773 |
13 ±
0.816497 |
13.5 ±
0.57735 |
14.75 ±
0.5 |
- |
| E. coli
(Gram -ve) |
24.51 ±
0.5 |
12.25 ±
0.5 |
13.25 ±
0.5 |
14.25 ±
0.5 |
15.25 ±
0.5 |
- |
EETC=Ethanolic extract of T. capensis, data are presented as Mean ± SD.
FIG. 7: ANTIBACTERIAL ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS. T1=50 mg EETC;T2=100 mg EETC;T3=150 mg EETC; T4=200 mg EETC; S=standard (Ciprofloxacin 50 µg/ml); C=Control (DMSO).
TABLE 3: ZONES OF GROWTH INHIBITION (mm) SHOWING ANTIFUNGAL ACTIVITY OF ETHANOLIC EXTRACT OF T. CAPENSIS; WELL DIAMETER 8.0 mm
| Name of the organism | Standard Fluconazole (0.1 mg/ml) | TI (50 mg of EEAN) | T2 (100 mg of EEAN) | T3 (150 mg of EEAN) | T4 (200 mg of EEAN) | Control |
| A. niger | 16 ± 0.11 | 10.5 ± 0.5773 | 11.5 ± 0.5773 | 12.5 ± 0.5773 | 14.25 ± 0.5 | - |
| C. albicans | 16 ± 0.16 | 12.25 ± 0.5 | 13.25 ± 0.5 | 14.25 ± 0.5 | 15.25 ± 0.5 | - |
EETC= ethanolic extract of T. capensis, data are presented as Mean ± SD.
FIG: 8: ANTIFUNGAL ACTIVITY OF ETHANOLIC EXTRACT OF T.CAPENSIS. T1=50 mg EETC; T2=100 mg EETC; T3=150 mg EETC; T4=200 mg EETC.
These infections can be treated with many standard drugs, but not all strains can be successfully treated by these drugs, further evaluation of this plant is needed. The extract shows moderate activity against microbes. This could be the beginning for further research on the screening approach by taking into consideration the extract preparation and the mechanism of action.
Anti-inflammatory Activity: Inflammation can be defined as a reaction of a living cell or tissue to injury, infection, irritation or infiltration. It is characterized by pain, swelling, redness and heat/ fever. Prostaglandins and leukotrienes are released by a host of mechanical, thermal, chemical, bacterial and other insults, and they contribute importantly to the signs and symptoms of inflammation 27.
TABLE 4: ANTI-INFLAMMATORY ACTIVITY OF TECOMA CAPENSIS CARRAGEENAN INDUCED RAT PAW EDEMA METHOD
| S. no. | Name of the sample | Dose
(mg) |
% inhibition of paw volume at various time intervals (hr) | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 1 | Control | ------- | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | Standard (Indomethacin) | 20 | 25.30 ± 0.41 | 42.90 ± 0.40 | 57.5 ±
0.43 |
63.09 ± 0.42 | 64.29 ±
0.31 |
72.35 ± 0.43 |
| 3 | Test extract -100
(EETC) |
100 | 20.64 ± 0.11 | 37.5 ±
0.12 |
45.94 ± 0.12 | 53.21 ± 0.11 | 56.36 ±
0.23 |
58.97 ± 0.11 |
| 4 | Test extract -250
(EETC) |
250 | 25.30 ± 0.12 | 40.58 ± 0.13 | 56.25 ± 0.14 | 62.48 ± 0.13 | 62.97 ±
0.12 |
71.73 ± 0.14 |
EETC=Ethanolic extract of Tecoma capensis, each value is presented as Mean ± SD of three values
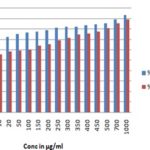
FIG. 9: ANTI-INFLAMMATORY ACTIVITY OF TECOMA CAPENSIS BY CARRAGEENAN INDUCED RAT PAW EDEMA METHOD. Data are represented as mean ± SD.
FIG. 10: ANTI-INFLAMMATORY ACTIVITY OF TECOMA CAPENSIS BY CARRAGEENAN INDUCED RAT PAW EDEMA METHOD. Data are represented as mean ± SD.
Mast cell which is very rich in histamine has membrane receptors both for a special class of antibody (IgE) and for complements components-C3a, and C5a mast cell can be activated to secrete inflammatory mediators through these receptors and also by direct physical damage. A transmembrane protein CD40 expressed on monocytes, neutrophils and platelets also play roles in inflammation 28. Our results clearly show the anti-inflammatory activity of the Tecoma capensis.
Generally, the effect of the ethanolic extract of Tecoma with inhibition ranging from 58.97 ± 0.11 to 71.73 ± 0.14, nearly equal to the standard Indomethacin. Mechanism possibly involved in this anti-inflammatory activity includes inhibition of the action of inhibitory mediators such as histamine, prostaglandins, nitric oxide, platelets activating factors and substance P, the effect on adreno corticoid hormone and immune suppression. This work is at present is limited to only animals; further clinical trials must require.
CONCLUSION: The results of the present study revealed that T. capensis extract possesses potent free radical scavenging ability, anti-inflammatory, and antimicrobial activity. The activity may be due to the presence of flavonoids, phenols, and tri-terpenoids. Further, we conclude that this plant can be used as a natural antioxidant, anti-inflammatory, and antimicrobial purpose.
ACKNOWLEDGEMENT: The authors are thankful for the management and staff members of the Raghu College of Pharmacy, Visakhapatnam for their constant support and providing me necessary facilities.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Jothi ET, Vimala DG, Vamsi AKC and Suba V: Toxicological studies and cytotoxic activity of ethanol and ethyl acetate extracts of Tecomaria capensis International Journal Research in Ayurveda and Pharmacy 2013; 4(3): 426-29.
- Iwaleva EO, McGaw LJ, Naidoo V and Eloff JN: Inflammation: the foundation of diseases and disorders, a review of phytomedicines of South African origin used to treat pain and inflammatory condition. African Journal of Biotechnology 2007; 6(25): 2868-85.
- Panduraju, Rao T and Kumar PRS: Antibacterial activity of Tecoma capensis methanolic extract of leaves. Asian Journalof Plant Science & Research 2011; 1: 102-115.
- Jothi ET, Ravichandiran V, Venkatesh P and Suba V: Anti-ulcer activity of different leaf extracts of Tecomaria capensis. International Journal of Pharmacyand Pharmaceutical Sciences 2012; 4(3): 709-713.
- Jothi ET, Durga P, Lakshmi THNV and Subha K: Investigation of preliminary phytochemical and anti-microbial activity of Tecomaria capensis (Thunb) Spach leaves extract. Pharmacologyonline 2011; 2: 1111-14.
- Neearj KS, Manmohan S and Birendra S: Evaluation of anti-pyretic activity of Tecomaria Capensis Pharmacologyonline 2011; 1: 712-16.
- Neeraj KS and Manmohan S: Anti-inflammatory, analgesic and antipyretic activity of methanolic Tecomaria capensis leaves extract. Asian Pacific Journal of Tropical Biomedicine 2012; 2(11): 870-74.
- Venkatesh P, Sriram P, Praveen P, Jothi E and Srinivasa P: Anti-inflammatory activity of leaf extract of Tecomaria capensis in rats. International Journal of Research in Ayurvedaand Pharmacy 2012; 4 (2): 265-66.
- Jothi ET, Ravichandiran V, Durga P, Srikanth V and Subha V: Hepatoprotective activity of different leaf extracts of Tecomaria capensis. International Journal of Pharmacyand Pharmaceutical Sciences 2012; 4(3): 252-56.
- Jothi ET, Vimala G, Vamsi CH and Subha V: Toxicological studies and cytotoxic Activity of ethanol and ethyl acetate extracts of Tecomaria capensis International Journal of Research in Ayurveda and Pharmacy 2013; 4 (3): 426-29.
- Armandodoriano B, Passacantilli and Giulianarighi: New iridoid glucosides from Tecoma capensis. Journalof Natural Products 1983; 46(3): 314-19.
- Molyneux P: The use of the stable free radical di-phenyl picryl hydrazyl (DPPH) for estimating antioxidant activity. SongklanakarinJournal of Science and Technology 2004; 26(2): 211-19.
- Sourav M, Bibhabasu H, Rhitajit S, Santanu B and Nripendranath M: Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista Evidence-Based Complementaryand Alternative Medicine 2011; 2011: 1-11.
- Mayank G and Manish KG: Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippenensisfruit extract on human erythrocytes: An in-vitro The Scientific World Journal 2014; 1-12.
- Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra RM and De Lourdes BM: Studies on the antioxidant activity of Lippia citriodora Infusion: Scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. Biological and Pharmaceutical Bulletin 2002; 25: 1324-27.
- Furuno K, Akasako T and Sugihara N: The Contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biological & Pharmaceutical Bulletin 2002; 25: 19-23.
- Puja S, Rashmi A, Santosh A, Arti S, Pratima S and Seema T: Optimization of antimicrobial activity of medicinal plants (Coriandrum sativum, Ocimum tenuiflorum and Phyllanthus emblica) against MDR pathogens. International Journal of Pharmaceuticaland Life Sciences 2013; 4(8): 2885-89.
- Turner RA: Screening methods in pharmacology. New York: Academic Press, Edition 1st, 1965.
- Mukesh G, Tejal S, Lal H, Asha P and Nayan P: Development and optimization of plant extract loaded nano emulsion mixtures for the treatment of inflammatory disorder. CurrentResearch in Drug Discovery 2014; 1(2): 29-38.
- Spencer JPE and Jenner A: Aroma of Intense oxidative DNA damage promoted by L-DOPA and its metabolites, implications for neurodegenerative disease. FEBS Letters 1994; 353: 246-50.
- Cheng Z, Li Y Chang W: Kinetic deoxyribose degradation assay and its application in assessing the antioxidant activities of phenolic compounds in a Fenton-type reaction system. Analytica Chimica Acta 2003; 478: 129-37.
- Pratapchandran R, Vysakhi MV, Manju S, Kannan M, Abdulkader S and Sreekumaran N: In-vitro free radical scavenging activity of aqueous and methanolic leaf extracts of Aegle tamilnadensis (Rutaceae). International Journal of Pharmacy and Pharmaceutical Sciences 2013; 5(3): 819-23.
- Zaidan MR, Noor Rain A, Badrul AR, Adlin A, Norazah A and Zakiah I: In-vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Tropical Biomedicine J 2005; 22(2): 165-70.
- Nakano MM, Zuber P: Anaerobic growth of a "strict aerobe" (Bacillus subtilis). Annual Review of Microbiology 1998; 52(1): 165-90.
- Ryan KJ and Ray CG: Sherris Medical Microbiology. McGraw hill, Edition 4th, 2004.
- Hossack DJN: Proteus vulgaris urinary tract infections in rats; treatment with nitrofuran derivatives. British Journalof Pharmacology 1962; 19: 306-312.
- Jasion DM and Jackson RI: Lipid-derived autocoids, eicosanoids and platelet-activating factor. Joel G H, Lee EL. Goodman & Gilman’s The Pharmacological Basis of Therapeutics: McGraw Hill, Edition 11th, 2006.
- Amazu LU, Azikiwe CCA, Njoku CJ, Osuala FN, Nwosu PJC and Ajugwo AO: Anti-inflammatory activity of the methanolic extract of the seeds of Carica papaya in experimental animals. Asian Pacific Journalof Tropical Medicine 2010; 3(11): 884-86.
How to cite this article:
Padmaja K, Seru G, Panda J and Sulakshna M: Antioxidant, anti-inflammatory and antimicrobial activity of whole plant of Tecoma capensis Thumb. Int J Pharmacognosy 2018; 5(2): 103-12. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(2).103-12.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
5
103-112
752
1709
English
IJP
K. Padmaja*, G. Seru, J. Panda and M. Sulakshna
Raghu College of Pharmacy, Dakamarri, Bheemunipatnam (M), Visakhapatnam, Andhra Pradesh, India.
pharma.padmaja@gmail.com
10 October 2017
14 November 2017
18 November 2017
10.13040/IJPSR.0975-8232.IJP.5(2).103-112
01 February 2018