ANTIMICROBIAL ACTIVITY OF MARINE SPONGE TETILLA DACTYLOIDEA: A COMPREHENSIVE PHYTOCHEMICAL, IN-VITRO, IN-SILICO, AND ADMET STUDY
HTML Full TextANTIMICROBIAL ACTIVITY OF MARINE SPONGE TETILLA DACTYLOIDEA: A COMPREHENSIVE PHYTOCHEMICAL, IN-VITRO, IN-SILICO, AND ADMET STUDY
S. M. Moazzem Hossen * and Mohammad Helal Uddin
Department of Pharmacy, Faculty of Biological Sciences, University of Chittagong, Chittagong-4331, Bangladesh.
Marine sponge (Tetilla dactyloidea) of the family Tetillidae is an unexplored medicinal sponge. This marine sponge from the Demospongia class, and its active ingredients have a number of possible medical uses. The study was for the assessment of Phytochemical, GC-MS, and antimicrobial potential of the acetone extract of Tetilla dactyloidea. Antimicrobial activity of T. dactyloidea acetone extract (25 µg) in terms of zone of inhibition (ZOI) varied from 9 ± 1.33 mm, 0 mm, 0 mm, and 0 mm while, Ciprofloxacin (5µg) showed ZOI ranged from 18 ± 1.35 mm, 16 ± 0.87 mm, 19 ± 1.16 mm, and 22 ± 1.22 mm against tested bacteria E. coli, S. typhe, P. aeruginosa, and S. aureus, respectively. Molecular docking demonstrated that compounds exhibited good binding affinity with the E. coli bacterial target. MD simulation demonstrated the stability of the top compounds complexed with the target proteins over 200 ns. All of the best compounds met the Lipinski rule and displayed traits found in medications. Thus, the current study suggests that the acetone extract of T. dactyloidea and its main phytocompounds can boost the bioactivity of its antibacterial action and may be a viable option for combating antibiotic drug resistance.
Keywords: Tetilla dactyloidea, Antimicrobial, antibacterial, molecular docking, molecular dynamics
INTRODUCTION: Infectious diseases now account for a growing portion of the world's health burden; they cause millions of deaths annually 1. One of the most important worldwide health concerns of the twenty-first century is thought to be bacterial infections 2. Several drug-resistant bacterial strains have emerged as a result of bacteria's evolution to avoid the effects of antibiotics.
The development of alternative and efficient anti-infective agents is a public health priority due to the growing antibiotic resistance of pathogens 3. Therefore, the need to discover novel antimicrobial agents is crucial, given the evidence of the fast global spread of resistant clinical isolates.
Nonetheless, the history of swift and extensive resistance to recently introduced antimicrobial agents suggests that even novel antimicrobial agent families will have a brief lifespan 4. The pharmaceutical industry uses a vast range of secondary products found in natural products derived from marine plants, either directly as predecessor or as top compounds 5. Numerous unusual living forms that may have unique chemical and biological properties and offer important therapeutic applications can be found in marine ecosystems, which are incredibly diverse. Because of their extraordinary biological activity and previously unheard-of sophisticated chemistry, sponges are among the most promising aquatic organisms 6. Several naturally occurring substances derived from ocean flora have been investigated and proven to be useful in their possible function as anti-infective against harmful microbes 7. These substances may have a therapeutic outcome in the treatment of contagious disease because they restrain bacteria via distinct pathway than traditional antibiotics 8. To date, marine sources have yielded a number of bioactive substances with different levels of action, including antitumor, anticancer, antimicrotubule, antiproliferative, cytotoxic, photoprotective, and antibiotic properties. The sponges (Phylum porifera) are still a rich source of new natural compounds with a wide range of distinctive biological activities among marine organisms. They are even thought to produce new marine natural products more frequently than anyone else 9, 10, 11, 12.
Tetilla dactyloidea (Carter, 1869) is a marine sponge that belongs to the class Demospongia. Recent research has shown that the active ingredients in this class have a number of possible medical uses 13. A dependable virtual method for finding, analyzing, and creating drugs and bioactive molecules with the highest level of accuracy is computer-aided drug design, or CADD. It can offer high throughput screening as quickly as possible while using less money and maintaining quality 14.
A useful technique in the drug discovery and development process, molecular docking has been repeatedly deployed to underscore the molecular interactions of ligands 15. It is one among numerous computational approaches designed to help researchers find and study new drug candidates 16. Therefore, a molecular docking evaluation was used to clarify the structural requirements for the interaction of bioactive compounds derived from sponges with antibacterial drug targets. With the aim of assessing the potential stability of the drug-protein complex, we performed a 200 ns MD simulation for each complex. Additionally, we examined the pharmacokinetics-toxicological profile and drug likelihood of the chosen compounds using ADME/T and Pass prediction tools, respectively. Our main purpose is to validate the antimicrobial activity of the marine sponge Tetilla dactyloidea using in-vitro and in-silico methods.
MATERIALS AND METHODS:
Collection and Preparation of the Extract Fraction: Sea sponge From the Gotivangha River, which runs between Moheshkhali and Sonadia Island, Tetilla dactyloidea was collected. Sponge collecting is followed by a seawater wash. After being sun-dried and ground, clean sponges are effectively extracted using acetone as a solvent.
Identification of Sample: The marine sponge was identified by Dr. M Shah Nawaz Chowdhury, associate professor, institute of marine science, University of Chittagong. The specimen number DP/CU/2021/01, deposited and preserved in Department of Pharmacy and Applied Chemistry & Chemical Engineering department. The identified marine sponge is Tetilla dactyloidea.
Preliminary Phytochemical Screening: Preliminary qualitative phytochemicals and secondary metabolites functional groups like alkaloids, flavonoid, tannin, glycosides, phenolic content, saponin content screening was carried out with the following standard protocols 17.
GCMS Analysis: Tetilla dactyloidea whole extract (ME-TD) was subjected to a gas chromatograph (Source: GC-17A, Shimadzu Corporation; stationary phase: silica capillary (Rxi-5 ms, 0.25 m, 30 m long, internal diameter: 0.32 mm) covered with DB-1 (J & W); mobile phase: helium gas; temperature: 70 °C (0 min), increasing to 150 °C at 10 °C/sec, hold time: 10 min, and the inlet temperature was maintained at 260 °C; pressure: 90 kPa; injection volume: 1 μL; flow rate: 0.6 mL/min); and mass spectrometer (MS, TQ 8040, Shimadzu Corporation, Kyoto, Japan). At 280oC, the temperature border between the GC and MS was kept unchanged. The MS ran in scan mode between 40 and 350 amu. Compound identification was carried out at the Institute of National Analytical Research and Service (INARS, ISO/IEC 17025:2017 accredited laboratory), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh, by comparing the sample with the GC-MS library (version 08-S) of the National Institute of Standards and Technology (NIST) 18.
Antimicrobial Activity Determination by Disc Diffusion Method: In this work, antibiotics were diffused into a nutrient-rich agar gel from a restricted source using the Disc Diffusion Method, which followed established protocols to produce a concentration gradient 19. There were discs with a common antibiotic (ciprofloxacin) and blank discs as positive and negative controls, respectively. Six-millimeter diameter nutrient agar discs were sterilized, dried, and then specific amounts of the test samples were applied. After then, these discs were incubated. The area surrounding the discs exhibits a zone of inhibition when exposed to conditions that prevent the growth of microbes. We acquired latest cultures of various bacterial isolates from the University of Chittagong's Department of Microbiology. After the discs were carefully deposited in the suitable wells on agar plates that had previously been inoculated with test bacteria, they were immersed in a 1 mg/ml sample solution (50 μl). The plates were kept in an incubator for around twenty-four hours at 37°C. The standard antibiotic ciprofloxacin was compared with the sponge extract's capacity to suppress bacterial growth, specifically the diameter of its zone of inhibition.
In-silico Investigation:
Ligand Preparation: 16 small molecules were identified from the GC-MS analysis of the acetone extract of T. dactyloidea sea sponge. These compounds were acquired from the PubChem database in 3D SDF format for docking purposes. If the 3D SDF format was unavailable, the 2D SDF format was downloaded and converted to 3D SDF using Open Babel software[20].Before docking simulation, all ligands were minimized and saved as .pdbqt format using AutoDock Tools (version 1.5.6) 21.
Protein Preparation: For antibacterial activity (PDB ID: 4KM2), was sourced from the RCSB Protein Data Bank (https://www.rcsb.org/structure) in PDB format. Utilizing Discovery Studio 2020 22 the protein structure was cleaned by erasing water molecules as well as other heteroatoms.
The protein was then subjected to energy minimization employing the steepest descent as well as conjugate gradient methods in Swiss-PDB Viewer (Version 4.1.0) 23. The PDB file was converted to PDBQT format with AutoDock Tools (version 1.5.6) as well and finally stored in this format.
Molecular Docking Analysis: The docking of the selected proteins with marine sponge ligands was executed utilizing PyRxAutoDock Vina 24. A semi-flexible docking system was used for this analysis, where the protein was stiff and ligands were flexible. AutoDock specified the parameters defining the box type as well as forming the grid box. The grid box was centered around the active site. Moreover, BIOVIA Discovery Studio Visualizer 2020 (Biovia, 2017) was employed to construct two and three-dimensional docking interactions.
ADMET Investigations: The pharmacokinetic properties (ADME) as well as toxicological attributes of the compounds were assessed using two online servers, SwissADME[25]and Pkcsm 26. Lipinski's Rule of Five 27 was considered for evaluating the positive drug-like attributes of the compounds.
Determination of PASS Prediction: The structure-activity relationship (SAR) analysis of a training set of more than 205,000 compounds was used to validate the PASS program, which predicts a compound's spectrum of activity as probable activity (Pa) and probable inactivity (Pi).This data was used to evaluate the chosen compounds' antibacterial efficacy 28. The values of Pi and Pa range from 0 to 1. If Pa > Pi, the compound is said to have experimental activity. In experiments, values below 0.5<Pa<0.7 indicate medium pharmacological activity, while values above 0.7 indicate strong pharmacological potentiality 29.
Molecular Dynamics Simulation: The protein-ligand system's structural stability was evaluated under a variety of circumstances using molecular dynamics (MD) simulations 30. The top two compounds were selected from the molecular docking study. GROMACS software was used to accomplish MD simulation on these compounds 31. MD simulation of the apo protein was also carried out to compare the level of protein stability with the resulting top-docked protein - ligand complexes. The SwissParam server 32 and the CHARMM 27 force field 33 were used to create the protein and ligand topology files, respectively. A triclinic box with a least distance of 1 nm from the box brink was used to solvate the protein using the TIP3P water model. The system was neutralized by adding two Na+ ions, and then it was minimized over 50,000 steps using the steepest descent algorithm.
Then, two-step equilibrations were carried out: NPT at 1 bar and NVT at 300 K. Each MD simulation was then run for 200 ns on the resultant system. Several GROMACS modules, such as the radius of gyration (Rg), solvent accessible surface area (SASA), number of hydrogen bond formations, root mean square deviation (RMSD), and root mean square fluctuation (RMSF), were employed to inspect the simulated trajectory for structural solidity.
Statistical Analysis: Data were analyzed as mean ± standard error of the mean (SEM). Statistical evaluations were conducted using one-way ANOVA followed by Dunnett's t-test. Differences from the control group were considered statistically significant at p < .001, p < .01, and p < .05. GraphPad Prism software (version 5.2) was used for all statistical analyses
RESULTS:
Preliminary Phytochemical Screening: According to the outcomes of preliminary qualitative phytochemical screening of marine sponge extract of Tetilla dactyloidea possesses a substantial quantity of important phytochemicals that may have potential health benefits. Glycosides, flavonoids, steroids, saponins, cholesterol, protein, and amino acids are just a few of the components that may be found within marine sponge Tetilla dactyloidea all the phytochemicals’ tests and results are tabulated in Table 1.
TABLE 1: PRELIMINARY PHYTOCHEMICAL SCREENING RESULTS
| Secondary Metabolite | Name of the test | Results |
| Alkaloids | Wagner's test | -- |
| Glycosides | General test | ++ |
| Cardiac glycosides | 1. Legals test | -- |
| 2. Baljet's test | -- | |
| Triterpenes | Salkowsky test | -- |
| Carbohydrate | Molisch's test | -- |
| Reducing Sugar | Benedict's test | -- |
| Flavonoids | 1. General test | ++ |
| 2. Specific test | ++ | |
| Steroids | Libermann- Burchard's test | ++ |
| Tannins | FeCl3test | -- |
| Saponins | Frothing test | ++ |
| Cholesterol | GCMS analysis | ++ |
| Proteins and Amino acid | Millon's test | ++ |
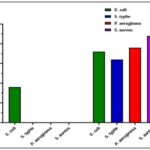
TABLE 2: ZONE OF INHIBITION FOR SEVERAL ORGANISMS BY DISC DIFFUSION METHOD
| Extract (25 µg) | Zone of inhibition (mm) | |||
| E. coli | S. typhe | P. aeruginosa | S. aureus | |
| MSAE | 9 ± 1.33 | - | - | - |
| Pefloxacin Standard (5µg) | 18 ± 1.35 | 16 ± 0.87 | 19 ± 1.16 | 22 ± 1.22 |
GCMS Analysis: All compounds given are identified by name as they showed more than 70% similarity when compared to the reference library. There were 16 compounds detected in the marine sponge extract of Tetilla dactyloidea. The major compounds are 9-Octadecene, (E)-, 2,6-Dimethyl-6-nitro-2-hepten-4-one, Hexanoic acid, heptadecylic ester, [1,1' Bicyclopropyl]-2-octanoic acid, 2'-hexyl, 1,37-Octatriacontadiene, (2R, 3R, 4aR, 5S, 8aS)-2-Hydroxy-4a, 5-dimethy, (2R, 3R, 4aR, 5S, 8aS)-2-Hydroxy-4a, 5-dimethy, (2R, 3R, 4aR, 5S, 8aS)-2 - Hydroxy - 4a, 5 - dimethy, Cholesterol, Ergost-5, 8(14)-dien-3-ol, Pregn-5-en-20-one, 3, 17-dihydroxy-, 3-acetat, beta-Sitosterol, (2R, 3R, 4aR, 5S, 8aS)-2-Hydroxy-4a,5-dimethy, (2R, 3R, 4aR, 5S, 8aS)-2-Hydroxy-4a, 5-dimethy, Spirost-8-en-11-one, 3-hydroxy-, (3.beta.,5.al).
FIG. 1: GCMS DATA OF MARINE SPONGE EXTRACT OF TETILLADACTYLOIDEA
FIG. 2: ANTIBACTERIAL ACTIVITY OF ACETONE EXTRACT OF MARINE SPONGE (MSAE) FOR SEVERAL ORGANISMS BY THE DISC DIFFUSION METHOD
Antimicrobial Screening: Antimicrobial activity was determined by disc diffusion method. The medium was Mullar Hilton Agar. The tested Bacteria was Gram Negative: E. coli, S. typhe and Gram Positive: P. aeruginosa, S. aureus. The Incubation period was 24-48 hours. Acetone extract exhibited the strongest activity against Escherichia coli and no discernible activity against the other test organisms. When compared to the standard Ciprofloxacin, the E. coli zone of inhibition was modest yet significant. Acetone extract of the marine sponge (MSAE) showed a zone of inhibition of 9 mm, while the standard Ciprofloxacin showed zone of inhibition 18 mm. Pefloxacin exhibits zone of inhibition of 16 mm, 19 mm, and 22 mm against S. typhe, P. aeruginosa, and S. aureus, respectively. But the acetone extract of marine sponge (MSAE) shows no inhibition to these organisms.
Docking Validation: The docking procedure was validated by comparing the reference ligand's lowest energy pose, which was obtained from Autodock Vina, to an experimentally determined binding pose using X-ray crystallography. The maximum reliability of the docking process is indicated by an RMSD of 0.232 Å between the docked pose and experimental pose, which is less than 2 Å 34. The identical conformations between the two poses can be observed by superimposing them shown in Fig. 3.
FIG. 3: SUPERIMPOSITION OF CO-CRYSTALLIZED LIGAND BEFORE (GREEN) AND AFTER (YELLOW) DOCKING (RMSD = 0.232 Å)
Molecular Docking Result of Antimicrobial Activity: Out of 16 marine sponge compounds, the best candidate was identified based on binding energy using molecular docking analysis. All of the substances and the reference molecule were docked into the E. coli dihydrofolate reductase binding site. Molecular docking revealed that four of the compounds in Table 3 had higher affinities for binding to the receptor than Ciprofloxacin, a standard compound with a binding energy of − 7.8 kcal/mol to the dihydrofolate reductase. Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha) from our natural marine sponge showed the highest binding affinity to the receptor, with a binding energy of − 9.7 kcal/mol. With a binding energy of -9.2 kcal/mol, beta-sitosterol, the second compound from our natural marine sponge, demonstrated the second-highest binding affinity to the receptor. Ergost-5,8(14)-dien-3-ol and Pregn-5-en-20-one, 3,17-dihydroxy-, 3-acetate are the third and fourth highest scoring compounds that exhibit binding energy -8.8 and 8.5 kcal/mol, respectively. Thus, in a subsequent investigation, we have exclusively focused on these three substances: Spirost-8-en-11-one, 3-hydroxy-, (3. beta., 5. alpha), beta-sitosterol, and Ergost-5, 8(14)–dien–3-ol. Table 3 and Fig. 4 provide a summary of the protein-ligand interaction analysis of the top three compounds and the standard molecule.
FIG. 4: MOLECULAR DOCKING INTERACTION OF COMPOUNDS AGAINST DIHYDROFOLATE REDUCTASE (PDB: 4KM2): (A1) SPIROST-8-EN-11-ONE, 3-HYDROXY-, (3.BETA., 5.ALPHA); (A2) BETA .- SITOSTEROL; (A3) ERGOST-5,8(14)-DIEN-3-OL; AND (A4) CIPROFLOXACIN (STANDARD)
Now, docking simulation showed that the standard molecule Fig. 4: a4 stabilized its protein-ligand complex by forming three hydrogen bonds: one conventional hydrogen bond with Ala-7 and two carbon-hydrogen bonds with Trp-6 and Gly-18. Additionally, it formed four hydrophobic bonds: one alkyl with Ile-94 and three pi-alkyl, as one with Phe-31 and two with Ile-20. Furthermore, it formed one halogen bond with Ile-14. In the same way, the top compound Spirost-8-en-11-one, 3-hydroxy-, (3. beta,5. alpha) Fig. 4: a1 showed that it can stabilize the protein-ligand complex by forming two carbon-hydrogen bonds: Ile-94 and Ile-5. Additionally, it formed six alkyl bonds: two with Ala-7, one with Met-42, one with Leu-50, one with Ile-94, and one with Ile-5. Furthermore, it formed two pi-alkyl bonds with Phe-31. Our second top compound, beta-sitosterol Fig. 4: a2, formed five alkyl bonds: one with Leu-57, one with Ile-94, two with Ile-14, and one with Ile-20. Additionally, it formed four pi-alkyl bonds with Phe-31. Our third top compound, Ergost-5,8(14)-dien-3-ol Fig. 4: a3, stabilized its protein-ligand complex by forming five alkyl bonds: one with Ala-7, one with Val-54, one with Leu-57, one with Ile-5,and one with Ile-14. Further, it formed four pi-alkyl bonds with Phe-31, and one with Tyr-100.
FIG. 5: MD SIMULATION RESULT OF DIHYDROFOLATE REDUCTASE (APO PROTEIN), APO PROTEIN-SPIROST-8-EN-11-ONE, 3-HYDROXY-, (3. BETA.,5. ALPHA) COMPLEX, APO PROTEIN-BETA. - SITOSTEROL COMPLEX, APO PROTEIN-ERGOST-5,8(14)-DIEN-3-OL COMPLEX, AND APOPROTEIN-STANDARD COMPLEX. (A) RMSD, (B) RMSF, (C) SASA, (D) RADIUS OF GYRATION, AND (E) INTERMOLECULAR HYDROGEN BONDS REPRESENT THE STRUCTURAL CHANGES AND FLEXIBILITY OF THE FIVE SYSTEMS. THE COLORS REPRESENTED APO-PROTEIN, APO PROTEIN-SPIROST-8-EN-11-ONE, 3-HYDROXY-, (3. BETA.,5. ALPHA) COMPLEX, APO PROTEIN-BETA. - SITOSTEROL COMPLEX, APO PROTEIN-ERGOST-5,8(14)-DIEN-3-OL COMPLEX, AND APOPROTEIN-STANDARD COMPLEX IN A, B, C, D, AND E ARE VIVID BLUE, ORANGE, ASH, YELLOW, AND SKY BLUE, RESPECTIVELY
TABLE 3: IN-SILICO ANTIMICROBIAL TARGET PROTEIN: DIHYDROFOLATE REDUCTASE (PDB: 4KM2)
| Compound Name | Binding Affinity | Hydrogen Bond Interactions | Hydrophobic Interactions | Halogen | |||||||||
| Conventional Hydrogen Bond | Carbon Hydrogen Bond | Alkyl | Pi-Alkyl | Pi-Sigma | |||||||||
| Amino Acid Residue | Distance (Å) | Amino Acid Residue | Distance (Å) | Amino Acid Residue | Distance (Å) | Amino Acid Residue | Distance (Å) | Amino Acid Residue | Distance (Å) | Amino Acid Residue | Distance (Å) | ||
| Ergost-5,8(14)-dien-3-ol | -8.8 | A: ALA7 | 4.22737 | A: PHE31 | 3.81477 | ||||||||
| A: VAL54 | 5.31086 | A: PHE31 | 3.98052 | ||||||||||
| A: LEU57 | 5.34058 | A: PHE31 | 5.21932 | ||||||||||
| A: ILE5 | 4.94637 | A: PHE31 | 4.83276 | ||||||||||
| A: ILE14 | 3.61567 | A: TYR100 | 5.2075 | ||||||||||
| Pregn-5-en-20-one, 3,17-dihydroxy-, 3-acetate | -8.5 | A: ARG60 | 2.57428 | A: TRP6 | 2.52211 | A: LEU57 | 4.9684 | A: PHE31 | 5.42563 | A: PHE31 | 2.72349 | ||
| A:PRO58 | 2.69289 | A: PHE31 | 4.01665 | ||||||||||
| A: PHE31 | 4.76224 | ||||||||||||
| beta. - Sitosterol | -9.2 | A: LEU57 | 5.26645 | A: PHE31 | 4.1363 | ||||||||
| A: ILE94 | 5.139 | A: PHE31 | 3.89302 | ||||||||||
| A: ILE14 | 4.03059 | A: PHE31 | 5.2451 | ||||||||||
| A: ILE20 | 4.72707 | A: PHE31 | 5.23536 | ||||||||||
| A: ILE14 | 4.54363 | ||||||||||||
| Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha) | -9.7 | A: ILE94 | 2.46202 | A: ALA7 | 4.66798 | A: PHE31 | 5.16443 | ||||||
| A: ILE5 | 2.58393 | A: ALA7 | 4.38539 | A: PHE31 | 4.83228 | ||||||||
| A: MET42 | 4.8769 | ||||||||||||
| A: LEU50 | 4.26782 | ||||||||||||
| A: ILE94 | 4.83711 | ||||||||||||
| A: ILE5 | 4.48797 | ||||||||||||
| Ciprofloxacin (Standard) | -7.8 | A: ALA7 | 1.81067 | A: TRP6 | 2.76688 | A: ILE94 | 4.69819 | A: PHE31 | 4.76614 | A: ILE14 | 3.3059 | ||
| A: GLY18 | 2.57898 | A: ILE20 | 5.24461 | ||||||||||
| A: ILE20 | 5.08992 | ||||||||||||
Assessment of Pharmacokinetic Profiles of Sponge Compounds: The pharmacokinetics results of GCMS scanned compounds are in Table 4. Our compounds demonstrate a good absorption profile. At the same time, they showed better distribution and excretion profiles according to the pharmacokinetic profile tool. Our top compounds showed no AMES or Hepatotoxicity.
TABLE 4: ASSESSMENT OF PHARMACOKINETIC PROFILES OF SPONGE COMPOUNDS
| Compound name | Solubility | GI absorption | BBB permeability | P-gp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | Log Kp (skin permeation) | PAINS | Synthetic accessibility | Total Clearance (log ml/min/kg) |
AMES Toxicity | Hepatotoxicity |
| 9-octadecene, (E) | 1.983 | No | No | ||||||||||||
| 2,6-dimethyl-6-nitro-2-hepten-4-one | Soluble | High | Yes | No | No | No | No | No | No | -6.21 cm/s | 0 alert | 2.6 | 0.653 | No | No |
| Hexanoic acid, heptadecyl ester | Poorly soluble | Low | No | No | Yes | No | No | No | No | -1.15 cm/s | 0 alert | 3.4 | 2.064 | No | No |
| [1,1'-Bicyclopropyl]-2-octanoic acid, 2'-hexyl- | Poorly soluble | High | No | No | Yes | No | Yes | No | No | -2.57 cm/s | 0 alert | 3.95 | 1.436 | No | No |
| (2R,3R,4aR,5S,8aS)-2-hydroxy-4a,5-dimethyl | Soluble | High | Yes | No | No | No | No | No | No | -5.35 cm/s | 0 alert | 4.45 | 2.495 | No | No |
| Cholesterol | Poorly soluble | Low | No | No | No | No | Yes | No | No | -2.47 cm/s | 0 alert | 5.98 | 1.19 | No | No |
| Ergost-5,8(14)-dien-3-ol | Poorly soluble | Low | No | No | No | No | No | No | No | -3.29 cm/s | 0 alert | 6.06 | 0.577 | No | No |
| Pregn-5-en-20-one,3,17-dihydroxy-,3-acetate | Moderately soluble | High | Yes | No | No | No | No | No | No | -6.11 cm/s | 0 alert | 5.18 | 0.623 | No | No |
| beta-Sitosterol | Poorly soluble | Low | No | No | No | No | No | No | No | -2.20 cm/s | 0 alert | 6.3 | 0.628 | No | No |
| Spirost-8-en-11-one,3-hydroxy-, (3 beta,5 alpha) | Moderately soluble | High | Yes | No | No | No | No | No | No | -5.94 cm/s | 0 alert | 6.7 | 0.256 | No | No |
PASS Prediction of GCMS Scanned Compound: The Pass prediction results of GCMS scanned compounds are in Table 5. The compound is considered to have experimental activity if Pa > Pi.Pi and Pa have values between 0 and 1. Our top compound Spirost-8-en-11-one,3-hydroxy-, (3 beta,5 alpha) exhibited Pa value 0.442, where the Pi value was 0.023. Further, the second top compound beta. – Sitosterol showed Pa value 0.283, while the Pi value was 0.066. Again, the third top compound Ergost-5,8(14)-dien-3-ol showed Pa value 0.184, while the Pi value was 0.133.
TABLE 5: PASS PREDICTION OF GCMS SCANNED COMPOUND
| Compounds | Biological Activity | |||||||||||
| Antioxidant | Antidiabetic | Antibacterial | Anthelmintic | Thrombolytic | Antiarthritic | |||||||
| Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | |
| 2,6-dimethyl-6-nitro-2-hepten-4-one | 0,285 | 0,026 | 0,249 | 0,076 | 0.378 | 0.036 | 0,292 | 0,059 | 0,230 | 0,039 | - | - |
| Hexanoic acid, heptadecyl ester | 0,210 | 0,050 | 0,190 | 0,177 | 0.168 | 0.034 | 0,483 | 0,017 | 0,258 | 0,021 | - | - |
| [1,1'-Bicyclopropyl]-2-octanoic acid, 2'-hexyl- | 0,140 | 0,115 | 0,144 | 0,062 | 0.218 | 0.103 | 0,203 | 0,096 | 0,273 | 0,016 | - | - |
| (2R,3R,4aR,5S,8aS)-2-hydroxy-4a,5-dimethyl | 0,162 | 0,088 | - | - | 0.455 | 0.021 | 0,264 | 0,069 | - | - | - | - |
| Cholesterol | 0,198 | 0,056 | 0,131 | 0,092 | 0.267 | 0.074 | - | - | 0,166 | 0,124 | - | - |
| Ergost-5,8(14)-dien-3-ol | 0,174 | 0,075 | - | - | 0.184 | 0.133 | - | - | - | - | - | - |
| Pregn-5-en-20-one,3,17-dihydroxy-,3-acetate | 0,203 | 0,053 | 0,138 | 0,075 | 0.202 | 0.115 | - | - | - | - | - | 0,342 |
| Beta-Sitosterol | 0,178 | 0,072 | - | - | 0.283 | 0.066 | - | - | - | - | - | 0,241 |
| Spirost-8-en-11-one,3-hydroxy-, (3 beta,5 alpha) | 0,244 | 0,038 | - | - | 0.442 | 0.023 | 0,202 | 0,096 | - | - | - | 0,411 |
Assessment of Drug Likeness Characteristics of Sponge Compounds: The drug likeness results of GCMS scanned compounds are in Table 6. There are five fundamental rules for a chemical compound to be a drug. The requisite traits are Molecular weight, No. H-bond acceptors, No. H-bond donors, Log Po/w, and No. of rotatable bonds, and these rules are called Lipinski rule of five. Our best compounds were able to adhere to the Lipinski rule of five.
TABLE 6: ASSESSMENT OF DRUG LIKENESS CHARACTERISTICS OF SPONGE COMPOUNDS
| Compound name | Molecular weight | No. H-bond acceptors | No. H-bond donors | Log Po/w | No. of
rotatable bonds |
TPSA | Lipinski
rule of five |
Veber rule |
| 2,6-dimethyl-6-nitro-2-hepten-4-one | 185.22 g/mol | 3 | 0 | 1.25 | 4 | 62.89 Ų | Yes | Yes |
| Hexanoic acid, heptadecyl ester | 354.61 g/mol | 2 | 0 | 7.74 | 21 | 26.30 Ų | Yes | No |
| [1,1'-Bicyclopropyl]-2-octanoic acid, 2'-hexyl- | 322.53 g/mol | 2 | 0 | 6.01 | 15 | 26.30 Ų | Yes | No |
| (2R,3R,4aR,5S,8aS)-2-hydroxy-4a,5-dimethyl | 234.33 g/mol | 2 | 1 | 2.75 | 1 | 37.30 Ų | Yes | Yes |
| Cholesterol | 386.65 g/mol | 1 | 1 | 6.75 | 5 | 20.23 Ų | Yes | Yes |
| Ergost-5,8(14)-dien-3-ol | 398.66 g/mol | 1 | 1 | 6.74 | 5 | 20.23 Ų | Yes | Yes |
| Pregn-5-en-20-one,3,17-dihydroxy-,3-acetate | 374.51 g/mol | 4 | 1 | 3.65 | 3 | 63.60 Ų | Yes | Yes |
| beta-Sitosterol | 414.71 g/mol | 1 | 1 | 7.24 | 6 | 20.23 Ų | Yes | Yes |
| Spirost-8-en-11-one,3-hydroxy-, (3 beta,5 alpha) | 428.60 g/mol | 4 | 1 | 4.3 | 0 | 55.76 Ų | Yes | Yes |
Molecular Dynamics Simulation Result: MD simulation is crucial for post-dock evaluation to investigate the nature of protein structure dynamics and time-dependent stability.MD simulations were run for 200 ns to interpret the degree of stability, flexibility, and binding behavior of the Dihydrofolate reductase (Apo protein), Apo-1st_compound complex, Apo-2nd_compound complex, Apo-3rd_compound complex, and Apo_standard complex. RMSD, RMSF, RG, SASA, and hydrogen bondwere the parameters obtained and analyzed following a 200 ns MD simulation trajectory. The graph produced from RMSD, RMSF, RG, SASA, and number of hydrogen bonds analysis are given in Fig. 5.
The RMSD quantifies the variation between the protein backbone's initial and final structural conformations. The stability of the protein's structure is evaluated using the differences observed during the simulation. Stable protein structures have the least variability in their protein backbones, while unstable protein structures have more variability. The RMSD values for the Cα backbones in each of the five systems were calculated for a 200 ns simulation. Fig. 5(A) shows that all five systems eventually stabilize. The average RMSD values for the Apo-protein, Apo-Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha)complex, Apo-beta. - Sitosterol complex, Apo-Ergost-5,8(14)-dien-3-ol complex, and Apo-Standard complex were 0.196 nm, 0.157 nm, 0.149 nm, 0.154 nm, and 0.159 nm, respectively.
The RMSF analysis can be used to determine which regions of proteins and protein-ligand complexes are rigid and flexible. The RMSF value was computed to assess the structural changes caused by ligand binding. The N-terminal of the amino acid showed greater flexibility in all three systems Fig. 5(B). The average RMSF values for the Apo-protein, Apo-Spirost-8-en-11-one, 3-hydroxy-, (3. beta, 5. alpha) complex, Apo-beta. - Sitosterol complex, Apo-Ergost-5,8(14)-dien-3-ol complex, and Apo-Standard complex were 0.099 nm, 0.083 nm, 0.087 nm, 0.082 nm, and 0.092 nm, respectively.
SASA was another important parameter that was examined in order to ascertain the extent to which water molecules could reach the protein surface mentioned in Fig. 5 (C). The Apo-protein, Apo-Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha) complex, Apo-beta. - Sitosterol complex, Apo-Ergost-5,8(14)-dien-3-ol complex, and Apo-Standard complex were demonstrated to have average SASA values of 92.79, 94.27, 93.88, 93.39, and 93.05 nm2, respectively. RG is used to assess the proteins' overall stability and compactness both before and after ligand binding during the simulation. The Apo protein had an average RG value of 1.579 nm, the Apo-Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha) complexhad 1.582 nm,Apo-beta. -Sitosterol complex had 1.579 nm, Apo-Ergost-5,8(14)-dien-3-ol complex had 1.581 nm, and the Apo-Standard complex had an average RG value of 1.575 nm, respectively. The RG values are denoted in Fig. 5 (D). The protein-ligand complex's stability is greatly aided by the hydrogen bond interactions.The average number of hydrogen bonds formed between Apo-Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha) complex, Apo-beta. - Sitosterol complex, Apo-Ergost-5,8(14)-dien-3-ol complex, and Apo-Standard complex 0.826, 0.145, 0.049, and 0.044, ranging from 1-5, 0-3, 0-2, and 0-2 hydrogen bonds, respectively are shown in Fig. 5 (E).
DISCUSSION: The initial qualitative phytochemical screening of marine sponge extract from Tetilla dactyloidea revealed a significant class of essential phytochemicals as well as secondary metabolites like glycosides, flavonoids, steroids, saponins, cholesterol, protein, and amino acids that could potentially provide health benefits. Literature also reveals that sponges fight for habitat, protect themselves from fouling, and ward off predators by producing secondary metabolites 35. These compounds are linked to several bioactivities, including common antibacterial and antioxidant properties 36. Gas chromatography- mass spectroscopy (GCMS) confirms the content of 16 phytochemicals through quantitative analysis in the acetonic marine sponge extract of Tetilla dactyloidea.
The sponge extract demonstrated antibacterial efficacy in experiments conducted on four distinct bacterial strains. The effectiveness of the test agents in terms of their antibacterial properties was assessed by their ability to hinder the growth of bacteria surrounding the discs, leading to the formation of a distinct area where bacterial growth is inhibited. The sponge extract, at a concentration of 25 µg/ml, exhibited highest levels of inhibition in Escherichia coli, closer to the Pefloxacin Standard (5 µg/ml). The sponge extract exhibited a zone of inhibition 9 mm in Escherichia coli while it comes to the standard the zone of inhibition is 18 mm. Pefloxacin exhibits zone of inhibition of 16 mm, 19 mm, and 22 mm against S. typhe, P. aeruginosa, and S. aureus, respectively while the sponge extract showed no activity against these organisms. This outcome demonstrated that the sponge extract exhibits potent antibacterial efficacy against E. Coli. The antimicrobial activity can be described to the bioactive constituents found in the sponge, such as flavonoids and steroids 37.
The in-silico molecular docking analysis provided support for the antimicrobial activity of the target protein dihydrofolate reductase (DHFR; PDB: 4KM2) of M. tuberculosis, which was expressed in Escherichia coli for the biologically active compoundsSpirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha); beta.- sitosterol; ergost-5,8(14)-dien-3-ol; and pregn-5-en-20-one, 3,17-dihydroxy-, 3-acetate showed the strongest docking interaction (-9.7 kcal/mol, -9.2 kcal/mol, -8.8 kcal/mol, and -8.5 kcal/mol respectively), which showed higher binding affinity with the reference medication ciprofloxacin (-7.8 kcal/mol), indicating the compounds' antibacterial activity. The M. tuberculosis DHFR can be targeted within the E. coli system because it is heterologously expressed in E. coli 38. Further, as anticipated, the closed state of M. tuberculosis DHFR is more effectively superimposed on the closed state of Escherichia coli DHFR 39.
Consequently, we targeted the M. tuberculosis DHFR to assess the antibacterial activity against E. coli. Out of 16 compounds, the top three compounds Spirost-8-en-11-one, 3-hydroxy-, (3. beta,5. alpha), beta-sitosterol, and Ergost-5,8(14)-dien-3-ol also showed the maximum binding interactions to the receptor molecule. The standard molecule showed three hydrogen interactions and four hydrophobic interactions with the receptor, while our top compound showed two hydrogen interactions and eight hydrophobic interactions with the receptor. Again, our second top compound showed nine hydrophobic interactions with the receptor. Further, our third top compound exhibited ten hydrophobic bonds with the receptor. This increase in the interactions with the receptor denotes the strong activity of the compounds. Molecular dynamics simulation further validates our experiment in a significant manner. The RMSD values for the Cα backbones in each of the five systems were calculated for a 200 ns simulation. Though a slight increase in RMSD was noticed after 50 ns and continued until the end of the simulation, the apo-protein remained stable throughout. The Apo-Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha) complex required 40 ns to reach full stability and remained stable for 200 ns.
It took 50 ns for the Apo-beta-sitosterol complex to stabilize completely, and it did so for 200 ns. Other complex also showed the stability over the whole simulation. Our top three compound showed minimum average RMSD than standard ciprofloxacin. In case of RMSF noticeable flexibility was observed at amino acid residues 16-20, 49-52, 68-71, 81-87, and 118-123 in the protein structure of all five systems. However, both three complexes of our top compound displayed significantly lower flexibility in the N-terminal region of the protein structures compared to the apo proteins. As a result, the binding of Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha), beta.-sitosterol, and Ergost-5,8(14)-dien-3-ol to the protein decreased its flexibility and gave it rigidity, suggesting that stable complexes were formed by these three marine sponge compounds fitting well at the protein's active site. In the RG analysis, it observed that all the five systems had similar average RG values.
Therefore, ligand binding never significantly altered the protein's compactivity, and all complexes were just as compact as apo protein. Reduced SASA values indicate more structural compactness because they indicate less protein expansion. It observed that the exposed surface area was quite similar to all the complexes with the protein. This finding implies that the exposed volume of the protein before and after the ligand binding do not differ significantly. Therefore, there was no significant growth in either of the protein-ligand complexes. The value of RG supports this conclusion. The protein complexes were comparatively compact and stable during 200 ns MD simulations, according to the SASA value and RG.The average number of hydrogen bond formed between the top three compound-apo complex is equal and more than the standard-apo complex. The overall hydrogen bond interaction indicates that all of the ligands, including Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha), beta.-sitosterol, and Ergost-5,8(14)-dien-3-ol create stable complexes with proteins. The PASS program forecasts the biological properties of small molecules by using structure-activity relationships from large collections of compound datasets. This tool indicated a higher probability of antibacterial agonist predictions (0.442/0.023) for our top compound spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha).
CONCLUSION: Tetilla dactyloidea acetone extract demonstrated strong antibacterial activity against gram-negative bacteria, E. Coli in the disc diffusion method. Also, Spirost-8-en-11-one, 3-hydroxy-, (3. beta.,5. alpha), beta.-sitosterol, and Ergost-5,8(14)-dien-3-ol were found to be among the most active compounds on E. coli strains' essential protein in-silico experiments. Additionally, the Molecular Dynamics simulation validated our docking experiments. The compounds passed the five rules of drug-likeness properties using in-silico ADME/T prediction, and also exhibited better PASS prediction value, allowing them to proceed with clinical trials and animal testing for potential use as a commercially valuable antibacterial agent. These results suggest that Tetilla dactyloidea, a marine sponge, may aid in the development of safe and effective antibacterial drugs. Also, this marine sponge can be an alternative treatment for M. tuberculosis. Pharmaceutical investigations will benefit from a more thorough investigation of these identified phytochemicals.
ACKNOWLEDGEMENTS: We highly acknowledge Ministry of Science and Technology, Bangladesh, Department of Applied Chemistry and Chemical Engineering, University of Chittagong, for research support.
CRediT Authorship Contribution Statement: S. M. Moazzem Hossen: Conceptualization, Project administration, Formal analysis, Investigation, Writing -review & editing; Mehnaz Kamal: Software, Validation, Writing – original draft. Neamul Hoque: Data curation, Visualization. Mohammad Helal Uddin: Conceptualization, Project administration, Writing -review & editing.
Data Availability Statement: All data generated or analyzed during this study are included in this manuscript.
Funding: No funding information is available.
CONFLICT OF INTEREST: Nil
REFERENCES:
- W. H. Organization, Global report on infection prevention and control 2024. World Health Organization 2024.
- Morris AK and Masterton RG: Antibiotic resistance surveillance: action for international studies. Journal of Antimicrobial Chemotherapy 2002; 49(1): 7–10.
- Thanigaivel S, Hindu SV, Vijayakumar S, Mukherjee A, Chandrasekaran N and Thomas J: “Differential solvent extraction of two seaweeds and their efficacy in controlling Aeromonas salmonicida infection in Oreochromis mossambicus: a novel therapeutic approach.” Aquaculture 2015; 443: 56–64.
- Marasini BP: “Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria.” Biomed Res Int 2015; 1: 265425.
- Shokeen P, Bala M and Tandon V: “Evaluation of the activity of 16 medicinal plants against Neisseria gonorrhoeae.” Int J Antimicrob Agents 2009; 33(1): 86–91.
- El-Demerdash A: “Chemical diversity and biological activities of marine sponges of the genus Suberea: A systematic review.” Mar Drugs 2019; 17(2): 115.
- Cowan MM: “Plant products as antimicrobial agents.” Clin Microbiol Rev 1999; 12(4): 564–582.
- Saad S, Taher M, Susanti D, Qaralleh H and Awang AFIBL: “In-vitro antimicrobial activity of mangrove plant Sonneratia alba.” Asian Pac J Trop Biomed 2012; 2(6): pp. 427–429.
- Bergmann W and Feeney RJ: “Contributions to the study of marine products. XXXII. The nucleosides of sponges. I.” J Org Chem 1951; 16(6): 981–987.
- Villa FA and Gerwick L: “Marine natural product drug discovery: Leads for treatment of inflammation, cancer, infections, and neurological disorders.” Immunopharmacol Immunotoxicol 2010; 32(2): 228–237.
- Mayer AMS, Rodríguez AD, Berlinck RGS and Fusetani N: “Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action.” Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2011; 153(2): 191–222.
- Blunt JW, Copp BR, Munro MHG, Northcote PT and Prinsep MR: “Marine natural products.” Nat Prod Rep 2006; 23(1): 26–78.
- Krishnan GS: “In-vitro, in-silico and in-vivo antitumor activity of crude methanolic extract of Tetilla dactyloidea (Carter, 1869) on DEN induced HCC in a rat model,” Biomedicine & Pharmacotherapy 2017; 95: 795–807.
- Baig HM: “Computer aided drug design: success and limitations.” Curr Pharm Des 2016; 22(5): 572–581.
- Azam F, Prasad MVV, Thangavel N, Shrivastava AK and Mohan G: “Structure-based design, synthesis and molecular modeling studies of thiazolyl urea derivatives as novel anti-parkinsonian agents.” Med Chem (Los Angeles) 2012; 8(6): 1057–1068.
- Hussain MS: “Structural, functional, molecular, and biological evaluation of novel triterpenoids isolated from Helichrysum stoechas (L.) Moench. Collected from Mediterranean Sea bank: Misurata-Libya.” Arabian Journal of Chemistry 2022; 15(6): 103818.
- Shaikh JR and Patil M: “Qualitative tests for preliminary phytochemical screening: An overview.” Int J Chem Stud 2020; 8(2): 603–608.
- Uddin MR: “Quality assessment of bottled and unbottled drinking water in Bangladesh.” Water (Basel) 2021; 13(15): 2026.
- Biemer JJ: “Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method.” Ann Clin Lab Sci 1973; 3(2): 135–140.
- O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T and Hutchison GR: “Open Babel: An open chemical toolbox.” J Cheminform 2011; 3: 1–14.
- Huey R, Morris GM and Forli S: “Using AutoDock 4 and AutoDockvina with AutoDockTools: a tutorial.” The Scripps Research Institute Molecular Graphics Laboratory 2012; 10550(92037): 1000.
- Sharma S, Sharma A and Gupta U: “Molecular Docking studies on the Anti-fungal activity of Allium sativum (Garlic) against Mucormycosis (black fungus) by BIOVIA discovery studio visualizer 21.1. 0.0,” Annals of Antivirals and Antiretrovirals 2021; 5(1): 28–32.
- Guex N and Peitsch MC: “SWISS‐MODEL and the Swiss‐Pdb Viewer: an environment for comparative protein modelling.” Electrophoresis 1997; 18(15): 2714–2723.
- Dallakyan S and Olson AJ: “Small-molecule library screening by docking with PyRx,” in Chemical biology: methods and protocols, Springer 2014; 243–250.
- Daina A and Zoete V: “Application of the SwissDrugDesign online resources in virtual screening.” Int J Mol Sci 2019; 20(18): 4612.
- Pires DEV, Blundell TL and Ascher DB: “pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures.” J Med Chem 2015; 58(9): 4066–4072.
- Chen X, Li H, Tian L, Li Q, Luo J and Zhang Y: “Analysis of the physicochemical properties of acaricides based on Lipinski’s rule of five.” Journal of Computational Biology 2020; 27(9): 1397–1406.
- Lagunin A, Stepanchikova A, Filimonov D and Poroikov V: “PASS: prediction of activity spectra for biologically active substances.” Bioinformatics 2000; 16(8): 747–748.
- Ahmed S: “In-vivo and in-vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in-silico approaches.” Clinical Phytoscience 2019; 5(1): 1–13.
- Shukla R and Tripathi T: “Molecular dynamics simulation of protein and protein–ligand complexes,” Computer-aided drug design 2020; 133–161.
- Abraham MJ: “GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers,” SoftwareX 2015; 1: 19–25.
- Bugnon M: “SwissParam 2023: a modern web-based tool for efficient small molecule parametrization.” J Chem Inf Model 2023; 63(21): 6469–6475.
- Bjelkmar P, Larsson P, Cuendet MA, Hess B and Lindahl E: “Implementation of the CHARMM force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models.” J Chem Theory Comput 2010; 6(2): 459–466.
- Acharya R, Chacko S, Bose P, Lapenna A and Pattanayak SP: “Structure based multitargeted molecular docking analysis of selected furanocoumarins against breast cancer.” Sci Rep 2019; 9(1): 15743.
- Proksch P: “Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs.” Toxicon 1994; 32(6): 639–655.
- Anteneh YS, Yang Q, Brown MH and Franco CMM: “Antimicrobial activities of marine sponge-associated bacteria.” Microorganisms 2021; 9(1): 171.
- Kumar MS and Pal AK: “A review of bioactive compounds from marine organisms with special mention on the potential of marine sponges in pharmacological applications.” Journal of the Marine Biological Association of India 2016; 58(1): 84.
- White EL, Ross LJ, Cunningham A and Escuyer V: “Cloning, expression, and characterization of Mycobacterium tuberculosis dihydrofolate reductase,” FEMS Microbiol Lett 2004; 232(1): 101–105.
- Dias MVB, Tyrakis P, Domingues RR, Leme AFP and Blundell TL: “Mycobacterium tuberculosis dihydrofolate reductase reveals two conformational states and a possible low affinity mechanism to antifolate drugs.” Structure 2014; 22(1): 94–103.
How to cite this article:
Hossen SMM and Uddin MH: Antimicrobial activity of marine sponge Tetilla dactyloidea: a comprehensive phytochemical, in-vitro, in-silico, and Admet study. Int J Pharmacognosy 2025; 12(6): 534-47. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.12(6).534-47.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
11
534-547
1333 KB
528
English
IJP
S. M. Moazzem Hossen * and Mohammad Helal Uddin
Department of Pharmacy, Faculty of Biological Sciences, University of Chittagong, Chittagong-4331, Bangladesh.
hossen.pharmacy@cu.ac.bd
05 June 2025
25 June 2025
27 June 2025
10.13040/IJPSR.0975-8232.IJP.12(6).534-47
30 June 2025