ANTIFUNGAL STUDY OF CHEMICAL CONSTITUENTS ISOLATED FROM PIPER CALDENSE C. D.C.
HTML Full TextANTIFUNGAL STUDY OF CHEMICAL CONSTITUENTS ISOLATED FROM PIPER CALDENSE C. D.C.
G. A. T. Silva 1, E. B. V. de Sousa 1, J. U. G. Jardim1, R. C. Montes * 1, B. F. Lira 2, J. Í. V. Costa 1, E. O. Lima 2, J. M. Barbosa Filho 1 and Maria C. O. Chaves 1
Department of Pharmaceutical Sciences 1, Department of Chemistry 2, Federal University of Paraíba, Universidade Federal da Paraíba, João Pessoa, 58051-900, Brazil.
ABSTRACT: An intensive phytochemical study of the two parts of the Piper caldense plant brings the isolation of secondary metabolites, generated by shikimate and the mevalonate pathways. Using chromatographic techniques, the ethanolic extract of leaves afforded two pheophytyns, the pheophytin (1), 151-hydroxy-pophyrinlactone a (2), a mixture of the β-sitosterol and stigmasterol steroids (3-4). Four derivatives of benzoic acid (5-8) and the trilinoein (9) were isolated from the fruits. The spectroscopic methods such as 1H and 13C Nuclear Magnetic Resonance (NMR) and electrospray mass spectrometry (ESI-MS) were used for structural characterization. The compounds were screened for their in-vitro antifungal activity against seven Candida strains. The control used in the antifungal assays was nystatin. In general, three compounds revealed fungal sensitivity, especially, the prenylated benzoic acid derivative (6) which accomplished the lowest minimum inhibitory concentration (MIC).
| Keywords: |
Piperaceae, Antimicrobial activity, Prenylhydroxybenzoic acid derivative, Antifungal activity
INTRODUCTION: The Piper Linn. gender comprises at least 1000 species and is the major gender of the Piperaceae family which have become source of specific classes of secondary metabolites with outstanding biological activities, including amides 1, 2, 3, 4 flavonoids 5, aristolactams 6, 7, 8, 9 propenylphenoles, terpenes, phenylalcaloids and benzoic acid derivatives 10, 11, 12, 13. The specie Piper caldense C. DC., popular known as “d’arda pepper,” is used in the state of Paraíba, Brazil, as sedative, antidote for snakebites and toothaches, as well as plaster on the affected area for pain relief 8.
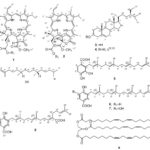
We report herein the isolation of natural compounds Piper caldense and the development of an antifungal study with these metabolites. These biocompounds were isolated from plant’s leaves and fruits and some of these chemical structures showed antimicrobial activity. All structures are shown on Fig. 1.
MATERIALS AND METHODS:
Experimental:
General Chemical Procedures and Instrumentation: The isolation and purification of the compounds were performed by chromatography column using gel silica 60 and Sephadex LH-20 (ART. 7734 Merck, Saint Louis, USA). It also used a medium pressure column in Pump Module C-605 equipment (Buchi, São Paulo, Brazil) with flash gel silica 60 (Merck, 230 - 400 mesh) as the stationary phase. The compounds were monitored by Thin Layer Chromatography (TLC) on silica gel aluminium plates 60 F254 (Merck), using ultraviolet light at two wavelengths (254 and 365 nm) from Mineralight apparatus (UVP, Upland, CA, USA) for detection. 1H and 13C NMR spectra were obtained in a Varian System machine (Palo Alto, USA) with 200 MHz and 500 MHz, using deuterated chloroform (CDCl3) as a solvent. Measurements of atomic mass for the compounds was carried out using a high-resolution mass spectrometer with ESI detector (Bruker, Massachusetts, EUA). The spectra were acquired in the detection mode of positive and negative ions, with the m/z range from 70 to 100.
Plant Material Treatment: The leaves and fruits of the Piper caldense C.DC (Piperaceae) were collected in Santa Rita-PB County, near the stream of the São João plant. The botanical identification was made by Maria de Fátima Agra, from PgPNSB/CCS/UFPB. A voucher specimen is archived in the herbarium Prof. LauroPires Xavier (CCEN/UFPB) with code Agra 20.311 ID.
Isolation of Chemical Constituents: The Piper caldense C. DC leaves were dehydrated in a stove with circulating air, at 40 ºC for 96 h. Then the 0,282 kg of powder was macerated in EtOH 95% for 72 h, yielding 50 g of crude ethanolic extract (EE). The EE was submitted to medium pressure chromatography with normal silica gel eluted with Hexane (Hex), ethyl acetate (AcOEt) and methanol (MeOH) as eluent and binary mixtures with increasing gradient polarity. Eight fractions were obtained and submitted to a chromatographic separations silica gel column.
The substances 1 (24 mg) and 2 (29 mg) was obtained from the fractions Hex: AcOEt (25:75) (4 g) and substance 3 and 4 (60 mg) from Hex: AcOEt fraction (1:1) (3 g). The same extraction procedure was made with the fruits of Piper caldense (950 g). An aliquot (5 g) of the hexanic extract was chromatographed and yielded the substances 4 and 5 from the elution with Hex: AcOEt (80:20) and substance 6 from the elution with Hex: AcOEt (60:40). The chromatographic separations of dichloromethane extract (16 g) yielded the compounds 7, 8 and 9.
Microbiological Strains: The microorganisms used in microbiological tests were strains of Candida albicans (ATCC 76645, LM-86 and LM- 111), C. krusei (ATCC-13803 and LM-18) and C. tropicalis (LM-08 and LM-13). The strains were respectively acquired from the Adolfo Lutz Institute in São Paulo (Brazil), and the Federal University Pharmaceutical Science Mycology Laboratories of São Paulo and Paraiba.
The yeast strains were maintained in an appropriate medium of sabouraud dextrose broth-SDB prepared and used according to manufacturer’s instructions (Difco Laboratories, Maryland, USA), and stored at 4 °C and 35 °C. The microorganism suspension was prepared according to McFarland tube 0.5 and adjusted using a spectrophotometer (Leitz-Photometer 340-800, Ernst Leitz, Wetzlar, Germany) at 90% T (530 nm) corresponding to approximately 106 CFU mL−1 [NCCLS, 2003; Hadacek, 2000; Eloff, 1978].
Determination of MIC: The MIC values were determined by the microdilution method using 96 well as “U” shaped microplates in duplicate. At each well of the plate, 100 µL of twice concentrated SDB liquid medium was added. Then, 100 mL of product solution (also doubly concentrated) was placed in the first row of plate wells. Through serial dilution (ratio of two), the concentrations of 1024 µg/mL to 16 µg/mL were obtained, such that in the first line of the plate was the highest concentration and at the latter were the lower concentrations. Finally, 10 µL of inoculum was added to the wells in each plate column that specifically referred to a strain. The same was also done in the culture medium with the fungal drug nystatin (100 IU).
The plates were incubated at 37 °C for 24 - 48 h. For each strain, the MIC was defined as the smallest concentration capable of inhibiting fungal growth in the wells as visually observed compared with the control. All tests were performed in duplicate, and the results were expressed as a geometric mean of the MIC values obtained in both tests. The antifungal activity of the products was interpreted, and considered active or not, according to the following parameters:
50 - 100 µg mL-1 = good activity; 100 - 500 µg mL-1 = moderate activity; 500 - 1000 µg mL-1 = low activity; greater than 1500 µg mL-1 = inactive 9, 10.
FIG. 1: CHEMICAL CONSTITUENTS ISOLATED FROM ETHANOLIC EXTRACT OF THE LEAVES AND THE HEXANE AND DICHLOROMETHANE EXTRACTS OF THE FRUITS OF PIPER CALDENSE C.DC. (A) PHYTYL CHAI
RESULTS AND DISCUSSION:
Chemistry: This study reports the isolation of two 151-hydroxi-porphyrin lactone (1) and pheophytin (2); a mixture of steroids: β-sitosterol (3) and stigmasterol (4); and, four prenylated derivatives of the benzoic acid (6 - 8) and also trilinoein (9). The structures of the compounds (1-9) were elucidated by 1H-NMR, 13C-NMR, HMQC, and HMBC spectra and their molecular formulas were established by ESI-MS and based on literature data 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21. The estimated purity values of compounds were at 92% - 96% calculated by 1H-NMR data 22. These natural constituents have been already reported in other botanic species of the Piper gender, such as the caldesinic acid (6) which has been isolated from aerial parts of Piper carniconnectivum 23 and from the Piper caldense C. DC. leaves 24.
In 1H-NMR (CDCl3, 500 MHz), for compound 6, it is possible to observe signals with chemical shifts in dH 7.87 (sl), 7.82 (dl, J=8.0 Hz) and dH 6.81 (d, J=8.5Hz), which indicates the presence of a trisubstituted ring. Signals with the chemical shifts in dH 5.33 (t, J=7.0 Hz), dH 5.08 (m), 3.37 (d, J=7.0 Hz), 2.18-2.08 (m), 2.07-2.00 (m), 1.98-1.94 (m), and singlet signals attributed to methyl groups - dH 1.75, 1.66, 1.59, 1.58 e 1.56 - suggest the presence of the geranylgeranyl group. In the HMBC spectra, it was observed the correlation of dH 6.81 (H-5) with δC121.26 and δC127.40, suggesting those signals are assigned to C-1 and C-3 positions, respectively. By correlating δH7.82 (H-6) with δC132.35, 159.54 and 171.75, it was possible to indicate the signal in δC132.35 to C-2 position and suggest δC 159.54 to C-4 position and δC 171.75 to the carbonyl group attached to C-1 position. The correlation dH 1.75 with δC 121.03, 138.48 and 39.68 allowed assign that the methyl group is attached to C-3’ and suggest the signal in δC 39.68 to the C-4’ position. The correlation dH 1.58 with δC 135.39, 39.65, 123.73 and dH 1.56 with δC 134.83, 124.37 and 39.60 allowed to solve the positions C-6’, C-7’, C-8’, C-10’, C-11’, C-12’, respectively, assigned to the signals in δC 123.73, δC 135.39, δC 39.65, δC 124.37, δC 134.83 and δC 39.60.
In the antifungal activity study, all compounds (1-9) were evaluated against Candida strains. The technique used was broth microdilution according to protocols 25, 26, using strains of Candida.
The control medium result showed no fungal growth, while the growth of fungi in the medium without any added drug (sterile control) was detected. The results are shown in Table 1. The MICs of the compounds were significantly different, ranging from 32 to 1024 μg.mL-1. The compounds which showed no inhibitory activity above 1024 μg.mL-1 were considered insignificant.
TABLE 1: QUANTITATIVE ANTIFUNGAL EVALUATION OF THE COMPOUNDS ISOLATED FROM PIPER CALDENSE WITH MICRODILUTION BROTH METHOD
| MICb (µg.mL-1) / Yeast | |||||||
| Compound | C. albicans
ATCC-76645 |
C. albicans
LM-86 |
C. albicans
LM- 111 |
C. tropicalis
ATCC-13803 |
C. tropicalis
LM-18 |
C. krusei
LM-08 |
C. krusei
LM-13 |
| 1 | 32 | + | 512 | 64 | 64 | + | 64 |
| 2 | + | + | + | + | + | + | + |
| 3/4 | + | + | + | + | + | + | + |
| 5 | 1024 | 512 | 512 | + | 32 | + | 256 |
| 6 | 256 | 32 | 32 | + | 32 | 256 | + |
| 7 | + | + | + | + | + | + | + |
| 8 | + | + | + | + | + | + | + |
| 9 | + | + | + | + | + | + | + |
+Growth of the microorganism; bMIC defined as the lowest concentration that produced 50% reduction infungal cell growth after 24 h of incubation
The pheophytin (1) inhibited the growth of five of eight (62.5% strains of Candida tested), and its smallest MIC was 32 µg.mL-1 to C. albicans ATCC-76645 similar to previous report 17. The second pheophytin (2) hasn’t inhibited any fungal strain, suggesting the porphyrinolactone ring decreases the inhibitory action in a pheophytin structure. The prenylated compounds 5 and 6 also inhibited the growth of 62.5% strains of Candida, and their smallest MIC was 32 µg/ml according to standard parameters 9, 10.
CONCLUSION: The study of leaves and fruits from the Piper caldense C. DC. (Piperaceae), manipulating usual chromatographic methods and spectroscopic techniques, have archived the isolation of nine natural compounds. Considering that investigation of fruits of the Piper caldense C.DC. is described for the first time in literature, a variety of structural compounds were isolated as two phfeophytins (1-2) and a mixture of steroids (3-4) from leaves. From the fruits of this Piper species were isolated five prenylated derivatives from the benzoic acid (5-8) and a one triglyceride (9). The compound 2 is reported in the Piperaceae family for the first time. In addition, pheophytin (1) and three prenylated compounds (5 and 6) exhibited significant antimicrobial activity against all yeasts tested.
Those results contributed to the chemotaxonomic of the Piper L. gender and of the investigated botanic specimen since there are few reports of studies with the Piper caldense C. DC.
ACKNOWLEDGEMENT: Authors are thankful to Universidade Federal da Paraíba. This work was supported by the following Brazilian agencies: CNPq and CAPES.
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES:
- Araujo-Junior JX, Da-Cunha EVL, Chaves MCO and Gray AI: Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry 1997; 44: 559-561.
- Chaves MCO, Júnior AGF and Santos BVO: Amides from Piper tuberculatum Fitoterapia 2003; 74: 181-183.
- Chaves MCO, Santos BVO and Oliveira AH: 1-cinnamoyl pyrrolidide from Piper marginatum. Biochemical Systematics and Ecology 2003; 31: 1213-1214.
- Santos BVO and Chaves MCO: (E,E)-N-Isobutyl-2,4-octadienamide from Piper marginatum. Biochemical Systematics and Ecology 1999; 27: 113-114.
- Alves HS, Oliveira GE, Zoghbi MG and Chaves MCO: Flavonoids from Piper carniconnectivum DC. (Piperaceae). Revista Brasileira de Farmacognosia 2010; 20: 160-164.
- AraújoJúnior JX, Chaves MCO, Cunha EVL and Gray AI: Cepharanone B from Piper tuberculatum. Biochemical Systematics and Ecology 1999; 27: 325–327.
- Cunha EVL and Chaves MCO: Two amides from Piper tuberculatum fruits. Fitoterapia 2001; 72: 197-199.
- Júnior ELC and Chaves MCO: Caldensin, A New Natural N-Methylaristolactam from Piper caldense. Pharma-ceutical Biology 2003; 41: 216-218.
- Aligiannis N, Kalpoutzakis E, Mitaku S and Chinou IB: Composition and antimicrobial activity of the essential oils of two Origanum species. Journal of Agricutral and Food Chemistry 2001; 49: 4168-4170.
- Santos BO: Reference method for broth dilution antifungal susceptibility testing of yeasts: NCCLS document M27-A3. In: National Committee for Clinical Laboratory Standards – NCCLS. Wayne, Pennsylvania, USA 2008; 1887-1898.
- Chaves MCO, Oliveira AH and Santos BVO: Aristolactams from Piper marginatum Jacq (Piperaceae). Biochemical Systematics and Ecology 2006; 34: 75-77.
- Chaves MCO and Santos BVO: Constituents from Piper marginatum fruits. Fitoterapia 2002; 73: 547-549.
- Santos BVO, Cunha EVL, Chaves MCO and Gray AI: Croweacin from Piper marginatum. Biochemical Systematics and Ecology 1997; 25: 471-472.
- Santos BVO, Cunha EVLC, Chaves MCO and Gray AI: Phenylalkanoids from Piper marginatum. Phytochemistry 1998; 49: 1381-1384.
- Santos BVO and Chaves MCO: 2, 4, 5-Trimethoxy-propiophenone from Piper marginatum. Biochemical Systematics and Ecology 1999; 27: 539-541.
- Smith KM, Goff DA and Abraham RJ: The NMR spectra of porphyrins. 27-proton NMR spectra of chlorophyll-a andpheophytin-a. Organic Magnetic Resonance 1984; 22: 779-783.
- Chaves OS, Gomes RA, Tomaz ACA, Fernandes MG, Mendes Junior LG, Agra MF, Braga VA and Souza MFV: Secondary metabolites from Sida rhombifolia (Malvaceae) and the vasorelaxant activity of Cryptolepinone. Molecules 2013; 18: 2769-2777.
- Maxwell A Sidarhombifolia Rampersad D: Novel prenylated hydroxybenzoic acid derivatives from Piper saltuum. Journal of Natural Products 1989; 52: 614-618.
- Flores N, Jiménez IA, Giménez A, Ruiz G, Gutiérrez D, Bourdy G and Bazzocchi IL: Antiparasitic activity of prenylated benzoic acid derivatives from Piper species. Phytochemistry 2009; 70: 621-627.
- Freitas GC, Kitamura ROS, Lago JHG, Young MCM, Guimarães EF and Kato MJ: Caldensinic acid, a prenylated benzoic acid from Piper caldense. Phytochemistry Letters 2009; 2: 119-122.
- Ragasa CY, Torres OB, Shen CC, Mejia MGR, Ferrer RJ and Jacinto SD: Chemical constituents of Aglaia loheri. Pharmacognosy Journal 2012; 4: 29-31.
- Malz F and Jancke H: Validation of quantitative NMR. Journal of Pharmaceutical and Biomedical Analysis 2005; 38: 813-823.
- Alves HS, Souza MFV and Chaves MCO: Caldensinic acid, a benzoic acid derivative and others compound from carniconnectivum. Química Nova 2010; 33: 802-804.
- Holetz FB, Pessini GL, Sanches NR, Cortez DAG, Nakamura CV and Filho BPD: Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias do Instituto Oswaldo Cruz 2002; 97: 1027-1031.
- Sartoratto A, Machado ALM, Delarmelina C, Figueira GM, Duarte MCT and Rehder VLG: Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Brazilian Jou Microbio. 2004; 35: 275-280.
- Houghton PJ, Howes MJ, Lee CC and Steventon G: Uses and abuses of in-vitro tests in ethnopharmacology: visualizing an elephant. Journal of Ethnopharmacology 2007; 110: 391-400.
How to cite this article:
Silva GAT, de Sousa EBV, Jardim JUG, Montes RC, Lira BF, Costa JIV, Lima EO, Filho JMB and Chaves MCO: Antifungal study of chemical constituents isolated from Piper caldensec D.C. Int J Pharmacognosy 2018; 5(8): 488-92. doi link: http://dx.doi.org/10.13040/ IJPSR.0975-8232.IJP.5(8).488-92.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
7
488-492
548
1550
English
IJP
G. A. T. Silva, E. B. V. de Sousa, J. U. G. Jardim, R. C. Montes *, B. F. Lira, J. Í. V. Costa, E. O. Lima, J. M. B. Filho and M. C. O. Chaves
Department of Pharmaceutical Sciences, Federal University of Paraíba, Universidade Federal da Paraíba, João Pessoa, Brazil.
ricsony_79@yahoo.com.br
15 May 2018
07 June 2018
13 June 2018
10.13040/IJPSR.0975-8232.IJP.5(8).488-92
01 August 2018