ANTIBACTERIAL ACTIVITY OF METHANOLIC EXTRACT OF MANGIFERA INDICA (BARK) AND OSYRIS LANCEOLATA (LEAVES) FROM WESTERN REGION OF NEPAL
HTML Full TextANTIBACTERIAL ACTIVITY OF METHANOLIC EXTRACT OF MANGIFERA INDICA (BARK) AND OSYRIS LANCEOLATA (LEAVES) FROM WESTERN REGION OF NEPAL
P. S. Bhandari 1, R. Bhandari 2, B. K. Sah 3, S. Gyawali 4, M. Bhusal 5, S. Shrestha 6 and S. Shakya * 7
Beximco Pharma Bangladesh Nepal Division 1, Bharatpur Chitwan, Nepal.
Department of Pharmacy 2, Crimson College of Technology, Pokhara University, Butwal - 13, Rupandehi, Nepal.
Koshi Zonal Hospital 3, Biratnagar, Morang, Nepal.
Lumbini Medical College 4, Palpa, Nepal.
Mercy City Hospital, Butwal 5, Rupandehi, Nepal.
Medical Biochemistry Department 6, Nobel College, Sinamangal, Nepal.
Market Planning Department 7, Everest Pharmaceuticals Pvt. Ltd, Tinkune, Koteshwor, Nepal.
ABSTRACT: Nature has been a source of medicinal agents since times immemorial. The importance of herbs in the management of human ailments cannot be overemphasized. Different extracts from traditional medicinal plants have been tested to identify the source of the therapeutic effects. As a result, some natural products have been approved as new antibacterial drugs. The objective of this research is to find out the potent antibacterial agents from the selected medicinal plants: Mangifera indica and Osyris lanceolata of Rupandehi, and Palpa districts of Nepal. The Bark and Leaves of the plants Mangifera indica and Osyris lanceolata respectively were subjected to extraction by maceration process using solvent methanol. Screening of antibacterial activity was done by disc diffusion method against the microorganisms Staphylococcus aureus and Escherichia coli at three different concentrations (1 mg/disc, 2 mg/disc, and 4 mg/disc). Both plants showed potent antibacterial activity. Minimum inhibitory concentration (MIC) assay was determined for the two extracts against these bacterias. The concentration 4 mg/disc of M. indica showed maximum activity against both E. coli and S. aureus, i.e., 11.16 ± 0.28 and 11.33 ± 0.29 respectively. Although both plants have exhibited best results against gram-positive as well as gram-negative bacteria, the crude extract of these plants further needs to be purified through antibacterial activity guided fractionation to isolate and identify the compounds responsible for the antibacterial activity. So, this study provides the platform to carry out further researches to give light upon specific compounds which are responsible for their antibacterial activities.
| Keywords: |
Antibacterial, Staphylococcus aureus, Escherichia coli, Methanol, Disc Diffusion Method, Minimum Inhibitory Concentration, Mangifera indica and Osyris lanceolata
INTRODUCTION: Medicinal plants contain physiologically active principles that over the years have been oppressed in traditional medicine for the treatment of various ailments as they contain anti-microbial properties 1.
Over the past 20 years, there has been an improved interest in the investigation of natural materials as sources of new antibacterial agents. The natural medicines are supposed to be more acceptable to the human body when compared to modern synthetic drugs 2.
Nepal is a Himalayan country with a great repository of natural products. So, there is a huge scope for the characteristic detailed study of such ethnobotanical plants having significant medicinal values in Nepal.
Although several plants with antimicrobial potential have been identified, the greater number remains unidentified. So, there is a dire need for proper evaluation of therapeutic properties of several other medicinal plants found in Nepal with a special reference to their ability to fight against various diseases 3. Mangifera indica was also known as an Aam or Mango is a large evergreen tree with a heavy dome-shaped crown and a straight stout trunk. It occurs throughout India, other parts of temperate Asia, southern Europe and America 4.
Various parts of Mangifera indica have been used in the indigenous system of medicine. The stem exudates (a gum resin) used in a dressing of cracked feet and scabies. It is also considered as antisyphilitic. The extract of various part exhibit moderate antibacterial activity against “Micrococcus pygenesvaraureus.” The bark also contains tannins (16-20%) and may be used for tanning purpose 5. The bark is used for many purposes such as a diuretic, astringent hemostatic and antirheumatic and also used in local hot baths and hot dressings. The bark is used in a wash for blennorrhea. The resin is useful in cutaneous disease. Various preparation of Gum resin from the bark is used in catarrh and mixture of gum resin and lime juice useful in scabies and other cutaneous infections.
A fluid extract from the bark is very useful in doses of one teaspoonful every hour or two mixed with two ounces of water in case of hemorrhage from the lungs, the uterus or intestine 6. Osyris lanceolata also known as Nundhiki or bark bush, is an evergreen hemi parasite multi-stemmed plant with a round to irregular canopy and a smooth grey bark that belong to the family Santalaceae. It is a large, slender hardy shrub or a small tree (7-10 m tall). The family host culturally and commercially important species that have been used for herbal medicine, religion, and perfumery oil industry. African sandalwood has wide ecological distribution and 300 species of plants from herbaceous, weed, grass, multi-stem shrubs and tree; a root decoction is used to treat diarrhea in Kenya; a decoction of the bark and heartwood is used to treat sexually transmitted diseases and anemia in Tanzania. Extracts from the plant can cure certain diseases, including the killer Hepatitis B. It was traditionally used by various Kenyan communities to preserve milk in gourds for long periods 7.
The microorganisms have developed resistance to many antibiotics because of indiscriminate use of antimicrobial drugs that create a big problem in the treatment of infectious diseases. With the increase in resistance of many microorganisms to the currently used antimicrobials and the high cost of production of synthetic compounds; in addition to many side effects; there is a need to look for the alternatives. Plants have provided a good source of anti-infective agents, antibacterial activity and remain highly effective instruments in the fight against microbial infections 4. Antibacterial agents are the substances produced by various species of microorganism (bacteria, fungi, actinomycetes) that suppresses the growth of other microorganisms and may eventually destroy those. When a new class of antibiotic is introduced, it is effective in the beginning, but will eventually select for survival of the small fraction of bacterial populations that have an intrinsic or acquired resistance mechanism 8.
This research aimed to evaluate the in vitro antibacterial activity of some selected ethnomedicinal plants from, Rupandehi, and Palpa districts of Nepal. This investigation may reveal the basis for new potent antibacterial medicines which would boost the commercial value of the medicinal plants. For this purpose, two medicinal plants were selected for the screening of antibacterial activity from methanolic extract against Staphylococcus aureus and Escherichia coli. It was carried out by taking the organic extracts of both the stem bark and leaf parts respectively of the plants at a different concentration, and their activities were recorded by estimating zones of inhibition as produced by a disc-diffusion method on Mueller-Hinton agar media.
MATERIALS AND METHODS:
Microorganisms Used in the Study: Staphylococcus aureus (S. aureus) is an opportunistic pathogen causing disease in human beings and animals. This microbe is a major cause in wound infections and sometimes leading to life-threatening diseases as osteomyelitis, endocarditis and toxic shock syndrome. The microbial cell surface as a whole displays unique molecular compositions made of lipids, carbohydrates, and proteins that may alter the mechanism by which peptide might interact with an epitope or receptor, thereby providing unique binding sites for the peptide interaction 9.
Escherichia coli (E. coli) is a gram-negative, facultative anaerobic and non-sporulating bacterium. Cells are typically rod-shaped and are about 2 micrometers (µm) long and 0.5 µm in diameter, with a cell volume of 0.6 - 0.7 µm 3. Optimal growth of E. coli occurs at 37 °C, but some laboratory strains can multiply at temperatures of up to 49 °C. This bacterium is commonly found in the lower intestine of warm-blooded organisms 10. Uncomplicated urinary tract infections caused by E. coli cause serious illness and death 11.
Antibiotics Used in the Study:
Ampicillin: Ampicillin is a β-lactam antibiotic which belongs to semi-synthetic penicillin. It inhibits bacterial growth by interfering with the transpeptidation reaction of bacterial cell wall synthesis. Resistance to ampicillin is due to inactivation of antibiotic by β-lactamase, modification of target PBPs, impaired penetration of drug to target PBPs and efflux 12.
Ciprofloxacin: Ciprofloxacin is one of the several new quinolone antibacterial agents that show broad antibacterial activity, low toxicity and potential for use as oral therapy in urinary tract as well as skin and soft infections. It is rapidly and well absorbed from the gastrointestinal tract 13. Ciprofloxacin targets the bacterial type II enzymes, DNA gyrase (GyrA and GyrB) and topoisomerase IV, and acts by stabilizing an intermediate stage of the DNA replication reaction thus inhibiting cell division 14.
Resistance to ciprofloxacin is caused by the changes in the amino acids sequences around the enzyme active site resulting in reduced drug affinity thereby allowing for the continued bacteria cell growth.
Solvents: The solvent used in the study was Methanol (Thermo Fisher Scientific, India Pvt. Ltd., Mumbai) and the Water used in the study was prepared in the laboratory with Distilled water plant.
Chemicals: The chemical used in the study were Mueller Hinton agar (MHA) (HiMedia Laboratories Pvt. Ltd., Mumbai) and Nutrient Broth (HiMedia Laboratories Pvt. Ltd., Mumbai).
Antibiotics: The antibiotics used in the study were Ampicillin AMP 10 (SD002-1PK) and Ciprofloxacin CIP10 (#SD080-1PK) which was obtained from Himedia Laboratory Pvt. Ltd. Mumbai – 400 086, India.
Equipments: Equipment used in the experiments were beakers (50 ml, 100 ml), volumetric flasks (500 ml, 1000 ml), micropipette (10 μl), pipettes (10 ml), round bottom flask (1000 ml), cotton, plastic bottle, aluminium foils, detergents, butter paper, conical flasks, measuring cylinders, spatulas (stainless steel), plantcutter, paper sheets, scissors, glass rods, washing brush, funnels, filter paper (Whatman no. 1), scale, map, marker, gloves, mask, mortar and pestle.
Instruments: Instruments used in the experiment were
- Autoclave (S.M. Scientific Instruments (P) Ltd., Delhi).
- Digital balance (ATX224, SHIMADZU Corporation, Philippines).
- A rotary evaporator (R-210/215, BUCHI Labortechok AG, Switzerland).
- Refrigerator (GL-M492YLG).
- Grinder and Distilled Water (DW) plant.
- Sonicator (INDOSATI Scientific Instruments (P) Ltd., Delhi).
- Hot air oven (S.M. Scientific Instruments (P) Ltd., Delhi).
- Incubator (S.M. Scientific Instruments (P) Ltd., Delhi).
Plant Materials: The plant materials were collected from Rupandehi and Palpa district. Scientific name and parts used of collected plant materials are given in Table 1 below.
TABLE 1: SELECTED PLANT MATERIALS
| S. no. | Scientific name | Parts used |
| 1 | Mangifera indica | Bark |
| 2 | Osyris lanceolata | Leaves |
Test Organisms: The test microorganisms used for this research were S. aureus (Gram-positive) and E. coli (Gram-negative) obtained from National Path Lab, Butwal, Rupandehi, Nepal.
Method:
Identification of Plant: The plants were identified with the help of Botanist (Bhavendra Niroula Ph.D. in botany from Bhagalpur University, India) and also compared with the kinds of literature.
Collection of Plant Parts: The plants were collected from Rupandehi and Palpa district, Lumbini Zone respectively Western Nepal. The selection of the species used in this study was mainly based on their ethnomedicinal evidence (literature) of use for a condition such as diarrhea, dysentery, skin disease, UTI, etc.
TABLE 2: DETAILS OF THE PLANT COLLECTION
| S. no. | Plant | Local | Parts | Collection | |
| Collection site | Collection date | ||||
| 1 | Mangifera indica | Aam | Bark | Rupandehi | September 2015 |
| 2 | Osyris lanceolata | Nundhiki | Leaves | Palpa | September 2015 |
Drying: Collected plant materials were cleaned with tap water and were then rinsed with distilled water. The remaining water was wiped with the help of the clean cloth. They were then air-dried in the shade under the newspaper at room temperature in a well-ventilated room. The drying was carried out for 15 days with proper checking at regular interval.
Grinding: After the plant parts were dried, they were ground to a fine powder using a portable grinding machine. The reduced powder mass was then passed through the sieve of mesh size 40. The sieved powder was kept in an airtight plastic bottle, sealed to prevent contamination and stored at room temperature in a dark place until use.
Extraction Procedure: For the extraction process, double maceration was carried out. During this process, the maceration of the herbs was carried out twice, and the total volumes of the menstruum to be used were divided into two parts in such a way that same quantity of menstruum was used for each maceration. For the first maceration, 50g of dried powder was allowed to remain in contact with the 250 ml of menstruum (i.e., methanol) with occasional shaking for 48 h. After the time was over the liquids were strained, and the marcs were pressed. The liquids were then filtered. The second part of the menstrum, i.e. remaining 250 ml was then added to the marcs and allowed to stand again for 48 h. Again the liquids were strained, and the marcs were pressed. The liquids obtained from both the maceration were combined and filtered through Whatman no. 1 filter paper.
Evaporation of Extracts: The filtrates obtained from the extraction process were then evaporated to dryness using rotary vacuum evaporator. The methanolic extracts were evaporated at a temperature of 30 °C. The gummy concentrate was kept in glass vials, and the percentage yields of the extract were calculated. Then, the gummy concentrate kept in vials was covered with aluminum foil and stored in the refrigerator at a temperature of 4 °C until use.
Anti-Bacterial Activity Test:
Preparation of the Solutions of the Extracts: Extract of both plant M. indica and O. lanceolata were weighed as 100 mg, 200 mg, and 400 mg separately. Then 100 mg of one plant was dissolved in 1 ml Dimethyl Sulphoxide (DMSO) by vigorous shaking and stirring which resulted in 100 mg/ml of solution. Then 10 µl of that solution was piped out and poured into the small sterile paper disc which resulted in 1mg/disc. Similarly 2 mg/disc and 4 mg/disc were prepared.
Preparations of Inoculums: Stock cultures were maintained at 4 °C on slopes of nutrient agar. Active cultures for experiments were prepared by transferring a loopful of cells from the stock cultures to test tubes of Mueller-Hinton broth (MHB) which was then incubated for 24 h at 37 °C in an incubator. The turbid solution of each bacterium was obtained and kept for further use. All these activities were carried out in horizontal laminar flow.
Antimicrobial Assay: Disc diffusion method was performed for antibacterial activity test. In-vitro antimicrobial activity was screened by using Mueller Hinton Agar (MHA). The MHA plates were prepared by pouring 15 ml of molten media into sterile Petri plates. The plates were allowed to solidify for 5 min and 0.1% inoculum suspension was swabbed uniformly, and the inoculum was allowed to dry for 5 min. The different concentrations of extracts (1, 2 and 4 mg/disc) were loaded on 5 mm sterile individual discs. The loaded discs were placed on the surface of the medium, and the compound was allowed to diffuse for 5 min, and the plates were kept for incubation at 37 °C for 24 h. The negative control was prepared using DMSO. Ampicillin (10 μg/disc) and Ciprofloxacin (10 µg/disc) were used as a positive control against gram-positive (S. aureus) and gram-negative (E. coli) bacteria respectively. At the end of incubation, inhibition zones formed around the disc were measured with a transparent ruler in millimeter. These studies were performed in triplicate 15.
Minimum Inhibitory Concentration (MIC) Assay: The MIC method was applied to extracts that proved their high efficacy against microorganisms by the turbidity method. The highest dilution of a plant extract that still retains an inhibitory effect against the growth of a microorganism is known as MIC. Selected plant extracts were subjected to a serial dilution (5 mg/ml to 0.1562 mg/ml) using sterile nutrient broth medium as diluents. 20 μl of an individual microorganism and 20 μl of selected plant extract were loaded in test tubes and inoculated at 37 °C for 24 h. The highest dilution of the plant extract that retained its inhibitory effect resulting in no growth (absence of turbidity) of a microorganism is recorded as the MIC value of the extract. A control experiment was run in parallel to study the impact of the solvent alone (without plant extracts) on the growth of the two test organisms 4.
RESULT AND DISCUSSION:
Extraction Yield: The crude drugs were extracted in methanol solvent. The extract yields of the crude drugs are given in the table.
Yield value of each extract was calculated as:
Yield value = Extracts obtained × 100% / The total amount of crude drug
TABLE 3: EXTRACTION YIELD PERCENTAGE
| S. no | Plants | Extract Yield %
(Methanol) |
| 1 | Mangifera indica (bark) | 5.56 |
| 2 | Osyris lanceolata (leaves) | 23.3 |
In our study, the methanolic extract of the bark of Mangifera indica shows 5.56% of yield value followed by 23.3% by methanolic extract of leaves of Osyris lanceolata.
Antibacterial Activity Test: The mean and standard deviation of the zone of inhibitions (in mm) were calculated and verified by using MS-Excel.
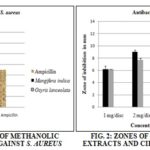
TABLE 4: ZONE OF INHIBITION OF METHANOL EXTRACT (IN mm) AGAINST S. AUREUS
| S. no | Extracts, Antibiotics, and Control | Zone of inhibition in mm against S. aureus | ||
| 1 mg/disc | 2 mg/disc | 4 mg/disc | ||
| 1 | Mangifera indica | 7 ± 0.5 | 10.33 ± 0.28 | 11.33 ± 0.29 |
| 2 | Osyris lanceolata | 6 ± 0 | 6.67 ± 0.29 | 9.67 ± 0.29 |
| 3 | Control DMSO | - | ||
| 4 | Ampicillin | 7 | ||
TABLE 5: ZONE OF INHIBITION OF METHANOL EXTRACT (IN mm) AGAINST E. COLI
| S. no | Extracts, Antibiotics, and Control | Zone of inhibition in mm against E. coli | ||
| 1 mg/disc | 2 mg/disc | 4 mg/disc | ||
| 1 | Mangifera indica | 6.17 ± 0.28 | 9 ± 0 | 11.17 ± 0.28 |
| 2 | Osyris lanceolata | 6.17 ± 0.28 | 7.67 ± 0.58 | 10.16 ± 0.28 |
| 3 | Control DMSO | - | ||
| 4 | ciprofloxacin | 13 | ||
The extract of Mangifera indica was used in the disc in the concentration of 1 mg/disc, 2 mg/disc and 4 mg/disc which shows the zone of inhibition against S. aureus in mm as 7 ± 0.5, 10.33 ± 0.28 and 11.33 ± 0.29 respectively. The extract of Osyris lanceolata was used in the disc in the concentration of 1 mg/disc, 2 mg/disc and 4 mg/disc which shows the zone of inhibition against S. aureus in mm as 6 ± 0, 6.67 ± 0.29 and 9.67 ± 0.29 respectively. The Ampicillin was used as a standard antibacterial agent which shows a zone of inhibition 7 mm against S. aureus. The DMSO was used as negative control which does not show any antibacterial activity.
The extract of Mangifera indica was used in the disc in the concentration of 1 mg/disc, 2 mg/disc and 4 mg/disc which shows the zone of inhibition against E. coli in mm as 6.17 ± 0.28, 9 ± 0 and 11.17 ± 0.28 respectively. The extract of Osyris lanceolata was used in the disc in the concentration of 1 mg/disc, 2 mg/disc and 4 mg/disc which shows a zone of inhibition against E. coli in mm as 6.17 ± 0.28, 7.67 ± 0.58 and 10.16 ± 0.28 respectively. The Ciprofloxacin was used as a standard antibacterial agent who shows the zone of inhibition 13 mm against E. coli. The DMSO was used as negative control which does not show any antibacterial activity. Extract of Mangifera indica showed a slightly greater zone of inhibition against S. aureus growth compared to the zone of inhibition against E. coli. While the extract of Osyris lanceolata showed a slightly greater zone of inhibition against E. coli growth compared to the zone of inhibition against S. aureus.
TABLE 6: MINIMUM INHIBITORY CONCENTRATION (MIC)
| Minimum inhibitory concentration | ||||||||||||
| Osyris lanceolata | ||||||||||||
| 5 mg/ml | 2.5 mg/ml | 1.25 mg/ml | 0.625 mg/ml | 0.3125 mg/ml | 0.1562 mg/ml | |||||||
| E. coli | - | - | - | - | + | + | ||||||
| S. aureus | - | + | + | + | ||||||||
| Mangifera indica | ||||||||||||
| E. coli | - | - | - | - | + | + | ||||||
| S. aureus | - | - | - | - | - | + | ||||||
(+) Represents growth of microorganisms
(-) Represent inhibition of growth of microorganisms
The Minimum Inhibitory concentration (MIC) was done which shows that 0.625 mg/ml and 1.25 mg/ml methanol extract concentration of Osyris lanceolata was sufficient to inhibit E. coli growth and S. aureus growth respectively. Similarly, methanol extracts concentrations 0.625 mg/ml, and 0.325 mg/ml of Mangifera indica were sufficient to inhibit the growth of E. coli and S. aureus respectively.
DISCUSSION: Recently much attention has been directed toward plant extract and biologically active compounds isolated from popular plant species. The use of medicinal plant plays a vital role in covering a basic health needs in developing countries, and these plants may offer new sources of antibacterial agents 16. The present study is focused on the antibacterial activity of two plants from Rupandehi and Palpa districts of Nepal. The Solvent methanol was used for extraction. It was seen that the extraction yield of Osyris lanceolata was higher than that of Mangifera indica.
The extraction yield for O. lanceolata was 23.027% and of M. indica was 5.56%.
In the present investigation, the antibacterial activity of plant extracts was tested against two microorganisms S. aureus and E. coli at three different concentration (1 mg/disc, 2 mg/disc, and 4 mg/disc) using disc diffusion method. All concentration showed potent antibacterial activity.
According to Doughari J H and Manzara S (2008), the antibacterial properties of crude leaf extracts of Mangifera indica against several bacteria was conducted among which the zone of inhibition against S. aureus was 0 mm, 3 mm, 5 mm, 7 mm and 9 mm and zone of inhibition against E. coli was 0 mm, 0 mm, 3 mm, 5 mm, and 7 mm at the concentration of 50 mg/ml, 100 mg/ml, 150 mg/ml, 200 mg/ml and 250 mg/ml respectively 17. Whereas in our study, the zone of inhibition against S. aureus was 7 mm, 10.33 mm, 11.33 mm, and zone of inhibition against E. coli was 6 mm, 6.67 mm, 9.67 mm at the concentration of 1mg/disc, 2mg/disc, and 4mg/disc respectively. The zone of inhibition shown in Doughari JH 17 studies was due to the components such as tannins, glycosides, saponin, and phenols. So, in our present study as well the antibacterial activity shown by the plants may be due to the presence of similar constituents.
In another study conducted by Wauthoz N et al.,18 an aqueous extract of M. indica reported containing mangiferin which has shown antibacterial activity against 7 bacteria among them S. aureus and E. coli was also taken. While in our study also the antibacterial properties of bark extract of M. indica suspected to contain mangiferin which showed a zone of inhibition against S. aureus and E. coli.
According to Yeboah EMO et al., (2013) 19 the microbial properties are shown from the root bark extract of Osyris lanceolata shown antimicrobial potential due to chemicals Dihydro-β- agarofuran. Similarly, in our study also an antibacterial property of methanolic extract of leaves of O. lanceolata may be due to the similar chemical compound. The polar organic compound may be present in the leaf extract of Osyris lanceolata which may have shown antibacterial agents S. aureus and E. coli.
In this present study the methanolic extract of M. indica in concentration of 1 mg/disc, 2 mg/disc and 4 mg/disc showed slightly higher antibacterial activity against S. aureus with the zone of inhibition (mm) of 7 ± 0.5, 10.33 ± 0.28 and 11.33 ± 0.29 respectively in compare to its zone of inhibition against E. coli i.e. 6.17 ± 0.28, 9 ± 0 and 11.17 ± 0.28 respectively. While the extract of O. lanceolata in the same concentration as above showed slightly higher antibacterial activity against E. coli with the zone of inhibition (mm) of 6.17 ± 0.28, 7.67 ± 0.58 and 10.16 ± 0.28 respectively compared to S. aureus with the zone of inhibition of 6 ± 0, 6.67 ± 0.29 and 9.67 ± 0.29 respectively. Both plants, with the increase in the concentration of the plant extract, showed a gradual increase in the zone of inhibition.
Our study also showed the microorganism used i.e., S. aureus was somewhat resistance to Ampicillin which showed only 7 mm of zone of inhibition which was lower than zone of inhibition of both plants extracts whereas the zone of inhibition of both plants at greatest concentration of 4mg/disc against E. coli was comparable to zone of inhibition of Ciprofloxacin, i.e. 13 mm. The Minimum Inhibitory concentration (MIC) was done which shows that 0.625 mg/ml and 1.25 mg/ml extract concentration of Osyris lanceolata was sufficient to inhibit E. coli growth and S. aureus growth respectively. Similarly, extract concentration of 0.625 mg/ml and 0.325 mg/ml of Mangifera indica was sufficient to inhibit the growth of E. coli and S. aureus respectively.
Our test result showed that gram-positive bacteria, i.e., S. aureus was more susceptible to selected plants extracts than gram-negative bacteria, i.e., E. coli. Various researchers have already shown that gram-positive bacteria are more susceptible to plants extracts as compared to gram-negative. These differences may be attributed to the fact that the cell wall in gram-positive bacteria is of single layer whereas cell wall gram-negative is multilayered structure 20.
CONCLUSION: Our results allow us to conclude that the crude extracts of both plants M. indica and O. lanceolata exhibited some extent of antibacterial activity. The results of the present study are encouraging as the tested extracts revealed antibacterial potential.
As both plants have exhibited antibacterial activity and shown the best results against gram-positive as well as gram-negative bacteria. It is therefore important to put out that the crude extracts of these plants need to be further purified through antibacterial activity guided fractionation to isolate and identify the compounds responsible for the antibacterial activity.
The results justify the use of these plants in folk medicine to treat various infectious diseases.
ACKNOWLEDGEMENT: The authors wish to thank Crimson College of Technology, Butwal-13, Devinagar, Rupandehi, Nepal, for providing the grant and facility to conduct this extensive project work and also take this opportunity to express gratitude to all of the Department faculty members for their help and support. We also place on record, our sense of gratitude to one and all, who directly or indirectly, have lent their hand in this venture.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Alla AA, Ishak CY and Ayoub MH: Antimicrobial Activity of four Medicinal Plants used by Sudanese Traditional Medicine. Journal of Forest Product and Industries 2013; 2(1): 29-33.
- Chandra M: Antimicrobial Activity of Medicinal Plants against Human Pathogenic Bacteria. International Journal of Biotechnology and Bioengineering Research 2013; 4: 653-658.
- Paudel PN and Gyawali R: Phytochemical Screening and Antimicrobial Activity of Some Selected Medicinal Plants of Nepal. International Journal of Pharmaceutical and Biological Archives 2014; 5(3): 84-92.
- Hussain AS, Mohammed Ali and Javed NK: Phytoconstituent from the Stem Bark of Mangifera indica variety “SAFEDA”. International Journal of Pharmacy 2011; 2(10): 103-105.
- Anjaneyulu V and Radhika P: The triterpenoids and Steroid from Mangifera indica Indian Journal of Chemistry 2000; 39(B): 883-893.
- Nikhal S and Mahajan SD: Evaluation of Antibacterial and Antioxidant Activity of Mangifera indica (leaves). Journal of Pharmaceutical Sciences and Res 2010; 2(1): 45-47.
- Stefanovic O, Radojevic I, Vasic S and Comic L: Antibacterial activity of naturally occurring compounds from selected plants. Laboratory of Microbiology, Department of Biology and Ecology, Faculty of Sciences, University of Kragujevac, Keragujevac Siberia 2004; 6: 1-24.
- Soares GMS, Figueiredo LC, Faveri M, Cortelli SC, Duarte PM and Feres M: Mechanism of action of systemic antibiotics used in periodontal treatment and mechanism of bacterial resistance of these drugs. Journal of Applied Oral Science 2012; 20: 295-309.
- Rao SS, Mohan KVK, Gao Y and Atreya CD: Identification and evaluation of a novel peptide binding to the cell surface of Staphylococcus aureus. Microbiological Research 2012; 2: 151-176.
- Marinez-Antonio A: Escherichia coli transcriptional regulatory network. Network Biology 2011; 1: 21-33.
- Vincent C: Food reservoir for Escherichia coli causing urinary tract infection. Emerging Infectious Diseases 2010; 16: 88-95.
- Katzung BG, Susan B and Trevor AJ: Basic and Clinical Pharmacology (Twelveth Edition) 2012; 790-792.
- Amini M, Khanavi M and Shafiee A: Simple high-performance liquid chromatography method for determination of ciprofloxacin in human plasma. Iranian Journal of Pharmaceutical Research 2004; 2: 99-101.
- Loveless BM: Identification of Ciprofloxacin Resistance by Simple Probe, high-resolution melt and Pyrosequencing Nucleic Acid Analysis in Biothreat agent: Bacillus anthracis, Yersinia pestis and Francisella tularensis. Molecular and Cellular Probes 2010; 24: 154-160.
- Shihabudeen MS, Priscilla H and Thirumurugan K: Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants. International Journal of Pharma science and Research 2010; 1(10): 430-434.
- Girish HV and Satish S: Antibacterial activity of important medicinal plants on human pathogenic bacteria-a comparative analysis. World Applied Sciences Journal 2008; 5(3): 267-271.
- Doughari JH and Manzara S: In-vitro antibacterial activity of crude leaf extracts of Mangifera indica African Journal of Microbiology Research 2008; 2: 067-072.
- Wauthoz N, Balde A, Balde ES, Damme MV and Duez P: Ethnopharmacology of Mangifera indica bark and pharmacological studies of its main c-glucosylxanthone, mangiferin. International Journal of Biomedical and Pharmaceutical Sciences 2007; 1(2): 112-119.
- Yeboan E and Majinda RRT: Five new Agaro furan sesquiterpene polyester from Osyris lanceolata. Research Gate 2013; 6: 531-535.
- Kajaria DK: Evaluation of Antimicrobial activity and bronchodilator effect of a polyherbal drug-shrishadi. Pacific Journal of Tropical Biomedicine 2012; 2: 905-909.
How to cite this article:
Bhandari PS, Bhandari R, Sah BK, Gyawali S, Bhusal M, Shrestha S and Shakya S: Antibacterial activity of methanolic extract of Mangifera indica (bark) and Osyris lanceolata (leaves) from western region of Nepal. Int J Pharmacognosy 2017; 4(6): 200-07. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.4(6).200-07.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
200-207
583
1262
English
IJP
P. S. Bhandari, R. Bhandari, B. K. Sah, S. Gyawali, M. Bhusal, S. Shrestha and S. Shakya *
Market Planning Department, Everest Pharmaceuticals Pvt. Ltd, Tinkune, Koteshwor, Nepal.
ssujyoti@gmail.com
03 March 2017
17 May 2017
30 May 2017
10.13040/IJPSR.0975-8232.IJP.4(6).200-07
01 June 2017