ANTIANXIETY, ANTICONVULSANT, MYORELAXANT AND HYPNOTIC EFFECT OF POLYHERBAL EXTRACTS ON RODENTS
HTML Full TextPOLYHERBAL EXTRACTS ON RODENTS
A. Tamil Selvan * 1 and R. Suresh 2
Department of Pharmacology 1, PSG College of Pharmacy, Peelamedu, Coimbatore - 614004, Tamil Nadu, India.
Department of Pharmacology 2, RVS College of Pharmaceutical Sciences, Sulur, Coimbatore - 641402, Tamil Nadu, India.
ABSTRACT: The research in alternative medicine and the need for the plant-based medicines that affect the ‘mind’ is developing day to day. The aim of the present study was to explore Leptadenia reticulata (roots), Mimusops elengi (bark) and Evolvulus alsinoides (Whole plant) using different animal models (conditional avoidance response, hole board test, open field test), anticonvulsant (MES induced convulsions), myorelaxant action (rotarod) and hypnotic property (Phenobarbital-induced sleeping) in suitable animal models. Diazepam (2 mg/kg, p.o), Phenytoin (25 mg/kg, p.o) and Phenobarbital sodium (50 mg/kg, i.p) were used as the standard drugs, and the acetone and ethanolic extract were 200 mg/kg, p.o Selected as per OECD guidelines. Results suggested that the extracts produced significant (p<0.001) anti-anxiety, myorelaxant and hypnotic action, but not significant anticonvulsant effect (p<0.05). Further studies are needed to identify the anxiolytic mechanism(s) and the phytoconstituents responsible for the observed CNS effects of the acetone and ethanolic extract of the selected plants.
| Keywords: |
Leptadenia reticulata, Mimusops elengi, Evolvulus alsinoides, Anxiolytic, Polyherbal, Diazepam
INTRODUCTION: Herbal remedies constitute a strong component of traditional, complementary and alternative medicine. In most developing countries, herbal remedies play a critical role in the management of various diseases owing to the challenges confronting the appropriate delivery of the official health care to millions of people in remote and rural areas 1. Anxiety affects one-eighth of the total population of the World and has become a very important area of research interest in psychopharmacology during this decade. Interest in alternative medicine and plant-derived medication that affects the mind is growing 2.
Anxiety, a state of excessive fear is characterized by motor tension, sympathetic hyperactivity, apprehension and vigilance syndromes 3. Leptadenia reticulata a much-branched twining shrub with yellowish-brown, corky and twining shrub with yellowish, cracked bark. The plant is a galactagogue, cooling, nutritive, aphrodisiac, stimulant, diuretic and eye tonic. It promotes health and vigor, improves voice and alleviates the three dosas vata, pitta, and kapha.
Mimusops elengi is a medium-sized evergreen tree found in tropical forests in South Asia, small, shiny, thick, narrow, pointed leaves, straight trunk and spreading branches. The bark, flowers, fruits, and seeds are astringent, cooling, anthelmintic, tonic and febrifuge. Evolvulus alsinoides this is a very slender, more or less branched, spreading or ascending, usually an extremely hairy herb. It is helpful in nervous exhaustion, memory loss, nootropic agent, general weakness, loss of memory, improves brain function like memory and concentration. Currently, much interest has been paid in search of medicinal plants with anti-anxiety action which may lead to the discovery of a new therapeutic agent that is not only used to suppress the anxiety but also used in the diverse disease conditions where the CNS amplifies the disease process. Bibliographical surveys showed that there is no report on the anti-anxiety activity of these above-selected combinations of the medicinal plants on the anti-anxiety, anticonvulsant, myorelaxant and hypnotic activities. This study was intended to evaluate the selected effects of the extracts experimentally by in-vivo in suitable models.
MATERIALS AND METHODS:
Plant Collection: Coarsely powdered materials of the plants Leptadenia reticulata (roots), Mimusops elengi (bark) and Evolvulus alsinoides (Whole plant) were collected from SKM Siddha and Ayurveda Company (India) Limited, Erode, Tamil Nadu, India.
Extraction: Equal amount (250 gm) of the weighed coarse powder of each plant part was mixed primarily and used for the extraction by successive solvent extraction by Soxhlet apparatus using various solvents (petroleum ether, chloroform, acetone, ethanol, and water - cold maceration). From the weight of each extractive residue, the extractive values were calculated in percentage. All the above extracts were used for the identification of constituents by preliminary phytochemical tests 4, 5.
Toxicity Study:
Animals: The experimental protocol was approved by the committee for control and supervision of experiments on Animals and Institutional Animal Ethics Committee (IAEC) Registration number (1012/c/06/CPCSEA) of the RVS College of Pharmaceutical Sciences, Sulur, Coimbatore - 641402. Swiss albino male mice weighing 20 - 25 gm and Albino Wistar rats of either sex weighing 160 -180 gm each was housed at 24 ± 2 °C with 12:12 h light and dark cycle. They had free access to food and water ad libitum. The animals were acclimatized for 7 days before the study. All the experiments were carried out between 10.00 to 16.00 h at ambient temperature. The animals were drawn at random for test and control groups.
Acute Toxicity Study: Acute toxicity study is generally carried out for the determination of LD50 value in experimental animals. The LD50 determination was done in mice by OECD guideline 423 acute oral class method. The aim of performing acute toxicity studies is for establishing the therapeutic index of a particular drug and to ensure safety. Adult albino mice (25-30 gm) were chosen for the study. They were maintained as per the standard laboratory conditions and provided with normal chow diet and water ad libitum.
At the day of the experiment the animals fasted for 12 h, and the extract was premixed with 1% gum acacia at the doses of Group I 5 mg/kg, Group II 50 mg/kg, Group III 300 mg/kg and Group IV 200 mg/kg were administered per orally (3 animals per group). Any changes in skin and eyes and mucous membrane and also respiratory, circulatory, autonomic, CNS, motor activity, behavioral pattern were observed. And also sign of tremors, convulsion, salivation, diarrhoea, lethargy, sleep, and coma was noted 6, 7. The abnormal signs of the animals were compared with the control group animals Group VI.
Conditional Avoidance Response: 8 The test cage is equipped with a single lever and a light. This cage is enclosed in a sound-attenuating chamber with a fan and with a speaker emitting a plain noise auditory background. The test cage has a grid floor of steel bars which are attached to a scrambled shock source. The data are recorded in an adjacent room. Mice with a starting weight of 18 - 25 gm are housed in individual cages. They are trained to avoid an unsignalled shock by repetitive lever-pressing responses.
A shock (1.5 mA for 0.5 s) is delivered to the grid floor every 15 s if no responses occur (shock-shock interval of 15 s = SS-15 s). A lever press (response) will delay the oncoming shock for 30 s (response-shock interval of 30 s = RS- 30s). The responses do not accumulate for delays of shock will be delivered 30 s after the last response is made even if 10 responses are made 31 s prior. Every 30 min, the total number of shocks received and the total numbers of responses made are accumulated and constitute the basic data. The animals are trained until they maintain a stable response rate and receive no more than 100 shocks/five h test session. After reaching these criteria of performance, experimental compounds are administered, and their effects on the performance of this learned avoidance behavior is evaluated. The experimental compounds or the standard are usually administered immediately before testing in volumes of 1 ml/kg of body weight. Depressant drugs lower the rate of lever presses and increase the number of shocks received. Stimulant drugs increase the rate of lever pressing. After each trial, the conditional avoidance response apparatus was wiped clean with ethanol (10%) solution.
Mice were treated with the extracts (200 mg/kg, p.o.) and vehicle for 7 days once daily p.o. and the last dose was given on the 7th day, 60 min before starting the experiment. The standard drug Diazepam was given at a dose of 2 mg/kg, p.o. 60 min before starting the experiment. For 1 min the total number of avoidance (lever-press/min) were measured. After each trial, the conditional avoidance response apparatus was wiped clean with ethanol (10%) solution. Group I: Animals received distilled water (1 ml/kg, p.o), Group II: Animals received diazepam (2 mg/kg, p.o.) in distilled water, Group III: Animals received Acetone extract of (200 mg/kg, p.o.) in distilled water, Group IV: Animals received ethanolic extract of (200 mg/kg, p.o.) in distilled water.
Hole Board Test: 8 The hole-board apparatus consists of a gray wooden box (40 × 40 cm × 2.2 cm thick) with 16 equidistant holes 3 cm in diameter in the floor. Animals were transported to the dim light laboratory used for this test at least 1 h before testing. Mice were treated with or distilled water 1 h before the testing animals. Each animal was placed singly in the center of the board facing away from the observer and its behavior recorded for 5 min; the number of head dips and rearing on the hole board were counted for 5 min. The latency to the first head dip was measured using a stopwatch. The duration of rearing, head-dip and spontaneous movements (number of squares crossed with all four paws) were also recorded.
Mice were treated with the extracts (200 mg/kg, p.o.) and vehicle for 7 days p.o. once daily the last dose was given on the 7th day, 60 min before starting the experiment. The standard drug diazepam was given at a dose of 2 mg/kg, p.o. 60 min before starting the experiment. The numbers of line crossing, numbers of head dipping and rearing were calculated for 5 min. After each trial, the hole-board apparatus was wiped clean with ethanol (10%) solution. Group-I: Animals received distilled water (1 ml/kg, p.o), Group-II: Animals received Diazepam (2 mg/kg, p.o.) in distilled water, Group-III: Animals received Acetone extract of C1 (200 mg/kg, p.o), Group-IV: Animals received ethanolic extract of (200 mg/kg, p.o.).
Open Filed Test: 8 The open field test, which provides simultaneous measures of locomotion, exploration, and anxiety, was used for this study. The open field is a 400 × 400 × 300 mm arena with thin black stripes painted across the floor; dividing it into 16 quadratic blocks. The mouse was placed in the center of the arena, and an observer quantified the spontaneous ambulatory locomotion of each mouse for 5 min. During this period, the number of squares crossed and several rearing were measured.
Mice were treated with the extracts (200 mg/kg, p.o.) and vehicle for 7 days p.o. once daily the last dose was given on the 7th day, 60 min before starting the experiment. The standard drug diazepam was given at a dose of 2 mg/kg, p.o. 60 min before starting the experiment. The numbers of line crossing, rearing, self-grooming, fecal droppings and activity in the center were calculated for 5 min. After each trial, the OFT apparatus was wiped clean with ethanol (10%) solution. Group-I: Animals received distilled water (1 ml/kg, p.o), Group-II: Animals received Diazepam (2 mg/kg, p.o.), Group-III: Animals received Acetone extract of (200 mg/kg, p.o.), Group-IV: Animals received ethanolic extract of (200 mg/kg, p.o.).
Anticonvulsant Activity: 8 In MES convulsions electroshock is applied through corneal electrodes. Through optic stimulation, cortical excitation is produced. The MES convulsions are divided into five phases (a) tonic flexion (b) tonic extensor(c) clonic convulsions (d) stupor and (e) recovery or death. A substance is known to possess anticonvulsant property if it reduces or abolishes the extensor phase of MES convulsions.
The anticonvulsant activity of the extracts was evaluated for maximum electroshock-induced seizure (MES) in the rat. The electrical shock applied (150 mA for 0.2 s) through corneal electrodes to Wistar Albino rats produced convulsion and those showing responses were divided into three groups of six animals each. The first group of animals was administered 1% normal saline (1 ml/kg) orally which served as a negative control. II group of animals were treated with phenytoin sodium (25 mg/kg, p.o.) which served as a positive control. Group III received acetone, and Group IV received the acetone extract at a dose of (200 mg/kg, p.o.). Group IV received ethanolic extract (200 mg/kg, p.o.). Drug pre-treatment was given 30 min before the electric shock and animal were observed for the duration of tonic, flexion, tonic extension, clonus and death/recovery.
Myorelaxant Property: 8 One of the important pharmacological actions of antianxiety agents of BZDs class of drugs is muscle relaxant property. The skeletal muscle relaxation together with a taming or calming effect, these agents reduce anxiety and tension. The loss of muscle-grip is an indication of muscle relaxation. This effect can be easily studied in animals using an inclined plane or rotating rods. Mice were divided into four groups consisting of six animals each. Group, I served as control which received distilled water. Animals of group II received standard drug diazepam at a dose of (2 mg/kg, p.o). Group III received acetone extract, and Group IV received ethanolic extract orally at a dose of 200 mg/kg, p.o. Animals are remaining on Rota-Rod (16 rpm) 2min or more in low successive trials after the administration of test material or control vehicle the same test of 30 min for 2 h. The fall off time from the rotating rod was noted. The difference in the fall off time from the rotating rod between the control and the treated rats was taken as an index of muscle relaxation.
Phenobarbital Induced Sleep Model: 8 Barbiturates induce sleep in man and animals by depressing the central nervous system. The barbiturate-induced hypnosis can be easily studied in experimental animals. For this purpose phenobarbitone sodium, a short-acting barbiturate is used. It produces the quick onset of sleep as indicated by loss of righting reflex (inability to maintain posture), and the recovery is also easily detected as the animals regain their righting reflex. Animals of Group I received distilled water, Group II received Phenobarbital sodium (50 mg/kg, i.p.), Group III received Phenobarbital (50mg/kg, i.p.) + acetone extract (200 mg/kg, p.o) and Group IV received Phenobarbital (50 mg/kg, i.p.) + ethanolic extract of at a dose of (200 mg/kg, p.o) respectively. The sleeping time of the Phenobarbital treated and sleeping time potentiated by the extracts treated animals were noted.
Statistical Analysis: Results were represented as mean + SEM. Data were analyzed using a statistical package (Graph pad prism version 3.00 to Windows, Graph pad software, San Diego, California, (USA). Comparison between groups was made using one-way analysis of variance (ANOVA) post-hoc comparisons were performed using Tukey-multiple comparison test.
RESULTS: From the weight of each extractor residue, the extractive values were calculated in percentage. The extractive value indicates the yield of the extract obtained from the air-dried plant powder by successive solvent extraction by Soxhlet extraction. Their percentage yield shows the solubility of the active principles in the organic solvents used based upon polar nature. They are then identified and confirmed by the preliminary phytochemical evaluation. The presence of phytoconstituents like alkaloids, flavonoids, carbohydrates, tannins, phytosterols, proteins, and amino acids, gums and mucilage, and resins are responsible for the typical pharmacological effects.
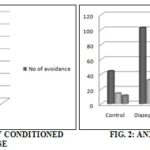
The LD50 determination was done in mice by OECD guideline 423 and LD50 of extracts were determined (infinity). In this study there was no toxicity/death were observed at the dose of 2000 mg/kg body weight in animals. The acute toxicity study showed that at 200 mg/kg dose the extracts are safe for consumption and medicinal uses. The therapeutic dose of the drug was considered as 1/10th of the LD50 value. Hence, the therapeutic dose used for recording biological response was 200 mg/kg, p.o for the extracts. The acetone and ethanolic extracts treated groups showed retention of conditioned avoidance response. Both the administration of the extracts significantly (p<0.001) increases the retention of conditioned avoidance response compared to standard.
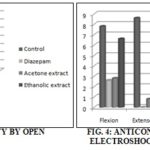
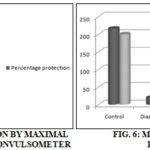
The results were shown in Table 1. The extracts showed an increase in number, latency, duration of head dipping and the number of rearing in the hole board. There was a significant (p<0.001) reduction in defecation units also seen with. The results were shown in Table 2. The total number of entries into the open field was statistically significant (p<0.001) when compared to standard drug diazepam treated animals. Animals treated with diazepam showed a slight decrease in the number of entries and the rearings was also reduced. The results were shown in Table 3. The results showed that the extractdid not affect stupor phase, but the animals get recovered at the earliest. Percentage protection showed less significant (p<0.05) when compared with the standard drug phenytoin. Hence, it may be of generalized tonic-clonic seizures, as it was a well-known fact that drugs which protect against seizures induced by MES were generally effective against generalized tonic-clonic seizures. The results were shown in Table 4 and 5.
TABLE 1: ANXIOLYTIC ACTIVITY BY CONDITIONED AVOIDANCE RESPONSE
| Treatment | No. of avoidance |
| Control | 3.48±0.35 |
| Diazepam (1mg/kg, i.p) | 6.03±0.70* |
| Acetone extract (200 mg/kg, p.o) | 5.30±0.30* |
| Ethanolic extract (200 mg/kg, p.o) | 6.14±0.45* |
n = 6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.001)
TABLE 2: ANXIOLYTIC ACTIVITY BY HOLE BOARD APPARATUS
| Treatment | No. of line crossing | No. of nose poking | Rearing |
| Control | 43.84±4.72 | 14.33±1.72 | 11.84±1.09 |
| Diazepam (1 mg/kg, i.p.) | 102.3±10.17* | 32.68±2.30* | 21.17±1.33* |
| Acetone extract (200 mg/kg, p.o.) | 73.00±6.21* | 25.33±3.20* | 17.50±1.30* |
| Ethanolic extract (200 mg/kg, p.o.) | 75.08±6.20* | 24.34±3.19* | 18.51±1.29* |
n=6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.001)
TABLE 3 ANXIOLYTIC ACTIVITIES BY OPEN FIELD TEST
| Treatment | No. of squares crossed | Rearing | Self-grooming | Activity in
center |
Ambulation |
| Control | 133.00±6.54 | 19.5±1.42 | 4.48±0.48 | 2.28±0.61 | 33.20±5.30 |
| Diazepam (1 mg/kg, i.p.) | 68.5±5.00* | 9.30±0.70* | 2.45±0.70* | 3.15±0.50* | 63.65±6.50* |
| Acetone extract (200 mg/kg, p.o.) | 50.00±9.96* | 9.48±0.73* | 3.75±0.62 | 5.60±0.64 | 86.20±10.80* |
| Ethanolic extract (200 mg/kg, p.o.) | 49.00±9.80 | 8.48±0.75 | 2.75±0.60* | 4.60±0.60* | 84.25±11.81 |
n = 6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.001)
TABLE 4: ANTICONVULSANT ACTIVITY BY MAXIMAL ELECTROSHOCK INDUCED BY CONVULSOMETER
| Treatment | Time(s) in various phases of convulsions | Recovery/
Death |
|||
| Flexion | Extensor | Clonus | Stupor | ||
| Control | 7.833±0.307 | 8.667±0.211 | 5.50±0.342 | - | Recovered |
| Phenytoin (25 mg/kg, p.o.) | 2.667±0.333* | - | 0.5±0.224 | 0.217±0.0833 | Recovered |
| Acetone extract (200 mg/kg, p.o.) | 2.8333±0.307* | 0.867±0.267* | 1.833±0.401* | - | Recovered |
| Ethanolic extract (200 mg/kg, p.o.) | 6.667±0.333 | 7.667±0.333 | 5.5±0.224 | - | Recovered |
n = 6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.05).
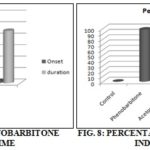
To verify the effect of the extract on muscle strength, Rota-Rod test was performed in mice. The acetone and ethanolic extract showed myorelaxant property significantly (p<0.05) compared to the standard drug. The results were shown in Table 6. At the dose level of 200 mg/kg, the acetone extract increased the duration of sleep. Meantime the potentiation the sleep potentiation of ethanolic extract was lesser than the acetone extract. The sign was found to be (p<0.001). The results were shown in Table 7 and 8.
TABLE 5: PERCENTAGE PROTECTION BY MAXIMAL ELECTROSHOCK INDUCED BY CONVULSOMETER
| Treatment | Percentage protection |
| Control | - |
| Phenytoin (25 mg/kg, p.o.) | 97.57%* |
| Acetone extract (200 mg/kg, p.o.) | 90.06* |
| Ethanolic extract (200 mg/kg, p.o.) | 11.54% |
n=6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.05)
TABLE 6: MYORELAXANT ACTIVITY BY ROTAROD APPARATUS
| Treatment | Fall of time(s) | |
| 15 min | 30 min | |
| Control | 216.667±13.824 | 200.667±6.339 |
| Diazepam (1 mg/kg, i.p) | 20.00±1.155* | 17.833±0.833* |
| Acetone extract (200 mg/kg, p.o.) | 99.00±3.00* | 97.667±3.159* |
| Ethanolic extract (200 mg/kg, p.o.) | 132.66±4.216 | 131.667±5.806 |
n=6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.05)
TABLE 7: POTENTIATION OF PHENOBARBITONE INDUCED SLEEPING TIME
| Treatment | Onset (min) | Duration of sleep (min) |
| Control | 9.155±0.182 | 72.5±1.176 |
| Phenobarbitone (50 mg/kg, i.p.) | 0.883±0.031* | 26.667±4.695* |
| Acetone extract (200 mg/kg, p.o.) | 2.66±0.112* | 104.333±1.647* |
| Ethanolic extract (200 mg/kg, p.o.) | 4.123±0.078 | 91.5±1.784 |
n = 6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.001)
TABLE 8: PERCENTAGE INCREASE PHENOBARBITONE INDUCED SLEEPING TIME
| Treatment | Percentage increase in sleep |
| Control | - |
| Phenobarbitone (50 mg/kg, i.p.) | 98.45%* |
| Acetone extract (200 mg/kg, p.o.) | 93.45%* |
| Ethanolic extract (200 mg/kg, p.o.) | 92.49% |
n=6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test.*(p<0.001)
DISCUSSION: The extracts prevented shock induced attenuation of the retention of conditioned avoidance response also. On the other hand, the extracts retarded the shock-induced enhancement of 5-HT mediated behavior. Extract-treated animals showed significant performance impairment in the conditioned avoidance response. Diazepam a putative anxiolytic drug increased the number of latency and duration of head dipping, and the number of rearing with the reduction in defecation units also noted. Therefore the increased number of head dipping and rearings represents a reflection of anxiolytic effect.
These results indicate that the effects were mainly mediated via the GABAnergic system and in part related to the highly sensitive benzodiazepine receptor-chloride ionophore complex 9. The OFT was done to determine the effect of the administration of the plant extracts upon spontaneous motor activity. There was decreased in locomotion activity in extract treated groups as the number of squares crossed in the perimeter was decreased between extract treated and control groups.
The extracts treated animals showed increased ambulation associated with no freeze and increased normal behavior like rearing and grooming associated with decreased defecation and urination. Many drugs that increase the brain content of GABA were exhibited anticonvulsant activity against seizures induced by MES. The acetone and ethanolic extract produced a moderate anti-convulsant action which was confirmed by the results obtained.
The extracts may be useful as an adjuvant therapy along with standard anticonvulsant requirements. It might be due to increased postictal depression, thereby indicating its CNS depression. GABA was the major inhibitory neurotransmitter, and the enhancement and inhibition of the neuro-transmission of GABA would attenuate and enhance convulsion respectively. The potential to improve psychomotor functions was one of the most common side effects of widely used sleep aids. The enhanced GABAergic transmission, the known mechanism for benzodiazepines, was associated with a loss of muscle strength, this finding suggests that the extract induced central nervous system related effects might be caused by the interference with the GABAA receptor. Benzodiazepines were known to facilitate GABA activate on of GABAA receptors intrinsic Chloride channel and in turn to facilitate inhibitory neurotransmission 10.
This was manifested as an increase in the frequency of ion channel opening in response to GABA. The effects might involve neurons that control central depressant activities. Because CNS depressant prolongs, barbiturate sleeping time can be said to display sedative property of the extracts. The effect was possibly mediated by positive modulation of the GABAA chloride channel receptor complex. Barbiturates appear to act primarily on the GABA: BZD receptor Chloride channel complex and potentiate GABAnergic inhibition by increasing the lifetime of chloride channel opening induced by GABA 11. BZDs which enhance the frequency of chloride channel opening. They induce hepatic microsomal enzymes and increase the rate of their metabolism as well as of many other drugs. Finally, the extracts treated animal showed increased sleeping time, which confirms the potentiation of phenobarbitone compared with the standard phenobarbitone. A dose-dependent effect on multiple neuronal targets appears to confer the ability to produce any grade of CNS depression.
CONCLUSION: The beneficial medicinal effects of plant materials typically result from the combinations of secondary metabolites present in the plant, through additive or synergistic action of several chemical compounds acting as single or multiple target sites associated with a physiological process. The findings of the present investigation indicated that the combinations of the plants Evolvulus alsinoides, Leptadenia reticulata, and Mimusops elengi extract studied for anxiolytic activity may be regarded as an anxiolytic agent view of its facilitatory effects on various animal models. Neurochemical basis of these extracts its inhibitory effect on brain serotonin and noradrenaline. However, further studies are necessary to confirm and extend these results. Thus the findings are relevant by contributing to our understanding of the traditional medical uses of these medicinal plants.
ACKNOWLEDGEMENT: Authors acknowledge and thank SKM Siddha and Ayurveda Company (India) Private Limited, Erode, Tamil Nadu, for their strong support in providing the herbal drug powders, lab facilities for extraction and phytochemical tests and the successful completion of the work.
CONFLICT OF INTEREST: The authors don’t have any conflict of interest in publishing this research work.
REFERENCES:
- Wambebe C: Development of Standardized phyto-medicines in Africa. J of Pharm Res and Develop 1998; 3: 1-11.
- Lader M and Morton S: Benzodiazepine Problems. Br J Addict 1991; 83: 823-828.
- Kokate CK: Practical Pharmacognosy. Vallabh Prakasham Delhi 1991; 5: 107-121.
- Olson H, Betton G and Robinson D: Concordance of toxicity of pharmaceuticals in humans and animals. Regulatory Toxicology and Pharmacology 2000; 5: 56-57.
- Kulkarni SK: Handbook of Experimental Pharmacology. Vallabh Prakashan, Delhi 1999; 3: 29-42.
- Vogel GH: Text Book of drug discovery and evaluation, pharmacological assays. Reck Publications & Distributors. Edition 2nd, 540-995.
- Bhattamisra: Anxiolytic activity of Marsilea minuta in rodents. J Herb Med Toxicol 2007; 1(1): 15-20.
- Weiss SM and Wadsorth G: Utility of ethological analysis to overcome locomotor confounds in elevated plus maze models of anxiety. Neuro Sci Behaviour Rev 1998; 2: 265-271.
- Tandon VR: An experimental evaluation of Anti-convulsant activity of Vitex negundo. Indian J Physiol Pharmacol 2005; 49(2): 199-205.
- Tripathi KD: Essentials of Medical Pharmacology. Jaypee Brothers Publishers, New Delhi, 2003; 399-402.
- Morpurgo C: Arzneim, Forsch. Drug Research 1971; 21: 1727.
How to cite this article:
Selvan AT and Suresh R: Antianxiety, anticonvulsant, myorelaxant and hypnotic effect of polyherbal extracts on rodents. Int J Pharmacognosy 2018; 5(8): 509-16. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(8).509-16.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
10
509-516
644
1261
English
IJP
A. T. Selvan and R. Suresh *
Department of Pharmacology, PSG College of Pharmacy, Peelamedu, Coimbatore, Tamil Nadu, India.
tamilselvanpharmacologist@gmail.com
17 May 2018
05 June 2018
13 June 2018
10.13040/IJPSR.0975-8232.IJP.5(8).509-16
01 August 2018