ANTI-INFLAMMATORY AND PHYTOCHEMICALS ANALYSIS OF CASSIA FISTULA LINN. FRUIT PULP EXTRACTS
HTML Full TextANTI-INFLAMMATORY AND PHYTOCHEMICALS ANALYSIS OF CASSIA FISTULA LINN. FRUIT PULP EXTRACTS
J. Anitha * and S. Miruthula
Department of Biotechnology, Arunai Engineering College Thiruvannamalai - 606603, Tamil Nadu, India.
ABSTRACT: Cassia fistula Linn. (Family Leguminosae, Subfamily Caesalpinia), a very common Indian plant is known for its medicinal properties. This plant is also known as Indian Laburnum, Yellow shower because of its characteristic yellow flowers in pendulous raceme and with typical branches. It is a native of Tropical Asia. It is widely cultivated in South Africa, Mexico, East Africa, and Brazil. This plant is used in folk medicine for tumors of the abdomen, glands, liver and throat cancer. It is also used to cure burns, constipation, convulsions, diarrhea, dysuria, and epilepsy. Ayurvedic medicines recognize as carminative and laxative. It is also used to cure leprosy, skin diseases and syphilis. Phytochemical investigations prove its importance as an important, valuable medicinal plant. C. fistula is known to be an important source of secondary metabolites notably phenolic compounds. It is known as a rich source of tannins, flavonoids, and glycosides. Pharmacological activities include antibacterial, antidiabetic, antifertility, anti-inflammatory antioxidant, hepatoprotective, antitumor, antifungal activities. This article aims to provide a comprehensive review of morphology, traditional uses, phytochemical constituents, and pharmacological activities.
| Keywords: |
Anti-inflammatory activity, Cassia fistula, Ethanol extract
INTRODUCTION: Inflammation: Cassia fistula L., (Fabaceae, Caesalpinioideae), a very common plant known for its medicinal properties is a semi-wild. It is distributed in various regions including Asia, South Africa, China, the West Indies, and Brazil. It is commonly known as Amultas and in English popularly called “Indian Laburnum” has been extensively used in Ayurvedic system of medicine for various ailments1-2. It is deciduous and mixed monsoon forests throughout greater parts of India ascending to 1300 m in outer Himalaya, is widely used in the traditional medicinal system of India.
Geographical distribution: In deciduous and mixed monsoon forests throughout greater parts of India, ascending to 1300 m in outer Himalaya. In Maharashtra, it occurs as a scattered tree throughout the Deccan and Konkan. The plant is cultivated as an ornamental throughout India 3.
Inflammation is defined as the local response of living mammalian tissues to injury due to an agent. It is a body defense reaction to prevent the spread of injurious agent and to remove the necrosed cells and tissues 4-5. The development of non-steroids in overcoming human sufferings such as Rheumatoid arthritis has evoked much interest in the extensive search for new drugs with this property. Inflammation can be classified as either acute or chronic. Acute inflammations the initial response of the body to harmful stimuli and is achieved by the increased movement of plasma and leukocytes (especially granulocytes) from the blood into the injured tissues. A cascade of biochemical events propagates and matures the inflammatory response, involving the local vascular system, the immune system, and various cells within the injured tissue 6. Five cardinal signs characterize it: The acronym that may be used for this is "PRISH" for Pain, Redness, Immobility (loss of function), Swelling and Heat 7.
The traditional names for signs of inflammation come from Latin-
Dolor (pain)
Calor (heat)
Rubor (redness)
Tumor (swelling)
Function laesa (loss of function).
Prolonged inflammation, known as chronic inflammation, leads to a progressive shift in the type of cells present at the site of inflammation and is characterized by simultaneous destruction and healing of the tissue from the inflammatory process.
Morphologic Patterns: Specific patterns of acute and chronic inflammation are seen during particular situations that arise in the body, such as when inflammation occurs on an epithelial surface, or pyogenic bacteria are involved.
Granulomatous Inflammation: Characterized by the formation of granulomas, they are the result of a limited but diverse number of diseases, which include among others tuberculosis, leprosy, sarcoidosis, and syphilis.
Fibrinous Inflammation: Inflammation resulting in a large increase in vascular permeability allows fibrin to pass through the blood vessels. If an appropriate procoagulative stimulus is present, such as cancer cells, a fibrinous exudate is deposited. This is commonly seen in serous cavities, where the conversion of fibrinous exudate into a scar can occur between serous membranes, limiting their function.
Purulent Inflammation: Inflammation is resulting in a large amount of pus, which consists of neutrophils, dead cells, and fluid. Infection by pyogenic bacteria such as staphylococci is characteristic of this kind of inflammation. Large, localized collections of pus enclosed by surrounding tissues are called abscesses.
Serous Inflammation: Characterized by the copious effusion of non-viscous serous fluid, commonly produced by mesothelial cells of serous membranes, but may be derived from blood plasma. Skin blisters exemplify this pattern of inflammation.
Ulcerative Inflammation: Inflammation occurring near an epithelium can result in the necrotic loss of tissue from the surface, exposing lower layers. The subsequent excavation in the epithelium is known as an ulcer.
Inflammatory Disorders: Inflammatory abnormalities are a large group of disorders which underlie a vast variety of human diseases. The immune system is often involved with inflammatory disorders, demonstrated in both allergic reactions and some myopathies, with many immune system disorders resulting in abnormal inflammation. Non-immune diseases with etiological origins in inflammatory processes include cancer, atherosclerosis, and ischaemic heart disease. A large variety of proteins are involved in inflammation, and any one of them is open to a genetic mutation which impairs or otherwise deregulates the normal function and expression of that protein.
Examples of Disorders Associated with Inflammation Include:
- Acne vulgaris
- Asthma
- Autoimmune diseases
- Celiac disease
- Chronic prostatitis
- Glomerulonephritis
- Hypersensitivities
- Inflammatory bowel diseases
- Pelvic inflammatory disease
- Reperfusion injury
- Rheumatoid arthritis
- Sarcoidosis
- Transplant rejection
- Vasculitis
- Interstitial cystitis
Cassia fistula: Cassia fistula, known as the golden shower tree. It is a popular ornamental plant and is herbal medicine. A medium-sized deciduous tree, 6-9 meters tall with a straight trunk and spreading branches. It is cold hardy and grows up to an elevation of 4,000 feet in the Himalayas. Stem bark is pale grey, smooth and slender when young and dark brown and rough when old. Leaves alternate 20-40 cm long, paripinnate, long-stalked stipulate petioles 6-9 mm long, leaflets 5-15 cm long, ovate, acute stalked. The flowers are lovely five-petaled with prominent, upward curving stamens. Fruit -a pod 30-60 cm long and over 30 cm thick. The tree has strong and very durable wood and has been used to construct"Ahala Kanuwa", a place at Adams Peak, Sri Lanka, which is made of Cassia fistula.
Scientific Classification:
Kingdom : Plantae
(Unranked) : Angiosperms
(Unranked) : Eudicots
(Unranked) : Rosids
Order : Fabales
Family : Fabaceae
Genus : Cassia
Species : C. fistula
Vernacular Name:
- Arabic : Khiār shambar
- Assamese : Xonaru
- Bengali : Sonalu, bandar lathi, amaltas
- Burmese : Ngu wah
- Chinese : Sausage tree
- Gujarati : Garmalo
- Hindi : Bendra lathi, dhanbaher
(dhanbohar), zirimaloah
- Hindi & Urdu : Amaltās
- Japanese : Nanban saikachi
- Khmer : Reachapreuk
- Kannada : Kakke
- Lao : Khoun
- Marathi : Bahava
- Malayalam : Kanikkonna (or kani
konna), Vishu konna
- Manipuri : Chahui
- Nepali : Amaltash, rajbriksya
- Sanskrit : Aragvadha, chaturangula,
kritamala, suvarnaka
- Sinhalese : Aehaela, (or ahalla), Ehela
- Tamil : Kondrai
- Telugu : Raela
- Thai : Rachapruek, khun, dok
Traditional Medicinal Uses: According to Hartwell, cassia fistula plants are used in folk remedies for tumors of the abdomen, glands, liver, stomach and throat cancer, carcinomata and impostumes of the uterus. Ayurvedic medicine recognizes the seed as antibilious, aperitif carminative and laxative the root for adenopathy, burning sensations, leprosy, skin diseases, syphilis and tubercular glands the leaves for erysipelas, malaria, rheumatism and ulcers the buds for biliousness constipation, fever, leprosy and skin disease the fruit for abdominal pain, constipation, fever, heart disease and leprosy. Unani use the leaves for inflammation the flowers for a purgative the fruit as anti-inflammatory, antipyretic, abortifacient, demulcent, purgative, refrigerant good for chest complaints eye ailments, flu, heart and liver ailments, and rheumatism, though suspected of inducing asthma. Seeds are considered asymmetric. In the West Indies, the pulp and leaves are poulticed onto inflamed viscera, e.g., the liver. The bark and leaves are used for skin diseases: flowers used for fever, root as a diuretic, febrifuge: for gout and rheumatism.
Phytochemical Constituents: The leaves of Cassia fistula contain free rhein, glucoside and sennosides A and B. A butanol extract of the powdered stem bark contained tannins while the benzene extracts yielded lupeol, b-sitosterol, and hexacosanol. Form the alcoholic extract of the pods an anthraquinone (fistulic acid) was obtained and identified as 1, 4-dihydroxy-6, 7-dimethoxy, 2-methylanthraquinone3 carboxylic acid, Kaempferol and a proanthocyanidin have been isolated from the flowers and leucopelargonidintrimer from the bark. The edible fruit tissue of (Cassia fistula L.), a member of the Leguminosae family, was analyzed for certain organic compounds and mineral nutrients. Of the nine macro- and micronutrients studied, K was the most highly concentrated such that 100% of the US Recommended Dietary Allowances (RDA) for adults could be met by the consumption of about 100 g of the fresh fruit. Na contents in pulp and seeds are relatively low. Ca content at 827 mg per 100 g of dry matter is one of the highest of any fruits and could contribute towards the RDA requirement of 800 mg of Ca for adults per day. The fruit is a good source of Fe and Mn, and their concentrations are considerably higher than those found in apple, apricot, peach, pear, and orange. Aspartic acid, glutamic acid, and lysine constituted 15.3, 13.0, and 7.8% of the total amino acids respectively in the pulp. In the seeds, the same amino acids constituted, 16.6, 19.5 and 6.6%. The relatively high energy content of the fruit at 18 kJ/g could enhance the daily energy requirement of people in need of adequate caloric intake.
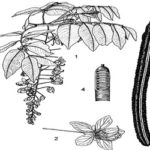
FIG. 3: 1- BRANCH WITH INFLORESCENCE 2- FLOWER 3- MATURE FRUIT 4-SECTION OF FRUIT SHOWING SEEDS
MATERIALS AND METHODS: The present study was carried out to evaluate the anti-inflammatory activity of Cassia fistula.
Qualitative and Quantitative analysis were done and the ethanolic extracts of the plant species was used for GC-MS studies. The details of the material used and methods followed are described below.
Collection of Plant Materials: Fruit pulp of Cassia fistula and were collected in the month of Jan-Feb, 2013 from local areas of Thiruvannamalai (Ramanaashramam). Fruit pulp of Cassia fistula were dried and finely powered and used for the study.
Chemicals and Reagents: Ethanol, Fehling’s reagent, Hydrochloric acid, sulphuric acid, Ferric chloride, acetic anhydride, chloroform, Mayer’s reagent, glacial acetic acid, ammonia, magnesium, Anthrone reagent, Bradford reagent.
Extraction: The plant materials were powdered, and 30 gm of powder sample was extracted with 150 ml of ethanol (1:5) by using sox let apparatus. The whole apparatus was kept over a heating mantle and was heated continuously for 24 h at the boiling point of the solvent. The extract was concentrated to dryness, and the residues were transferred to a pre-weighed sample bottle and were stored in desiccators for further studies.
Qualitative Analysis: Different biochemical parameters like reducing sugar, Flavonoid, Terpenoid, Tannin, Saponin, Anthraquinone, glycosides, alkaloids, etc. were tested.
Test for Reducing Sugars: The aqueous extract was added to boiling Fehling solution in a test tube; a brick red color indicates the presence of reducing sugars.
Test for Flavonoids: The extract and add a few magnesium turnings, followed by the addition of con. HCl drop by drop. A pink color indicates the presence of flavonoids.
Steroids and Terpenoids: Extract, dry and dissolve in chloroform. Add a few drops of acetic anhydride and conc. H2SO4 and keep undisturbed for few minutes. Formation of green color indicates the presence of steroids, while pink color indicates the presence of terpenoids.
Test for Tannins: To extract, add 2 drops of 5% FeCl3. Presence of dirty green precipitate indicates the presence of tannin.
Test for Saponin: To extract was shaken with 5 ml of distilled water and was heated to the boiling point. Frothing indicates the presence of saponin.
Test for Anthraquinones: To powdered material add 10 ml of 1% HCl and boiled for 5 min. Filter the sample and allowed to cool. Partition the cool filtrate against an equal volume of chloroform. Carefully transfer the chloroform layer into clean test tubes. Shake with an equal volume of 10% ammonia solution and allow the layer to separate. Presence of delicate rose pink color indicates the presence of combined anthraquinones.
Glycosides: To 0.5 gm of extract diluted to 5 ml with distilled water and add 2 ml of glacial acetic acid and containing one drop of ferric chloride solution. This was underplayed with 1ml of conc. H2SO4. Brown ring at the interface the presence of glycosides.
Test for Alkaloids: 5 ml of extract evaporated to dryness. Residue heated on a boiling water bath with 2% HCl. Then filtered, treated Mayer’s reagent. The yellow precipitate is indicating the presence of alkaloid.
A quantitative Test for Cassia fistula:
Determination of Moisture: 5 gm of material was taken in a pre-weighed Petri dish. The Petri dish was placed without lid into an oven at 110 ºC for three hours. The Petri dish was taken out and closed immediately with a lid. The dish was cooled in a desiccator and weighed. The amount of moisture of the material was calculated from the difference in weight.
Total Carbohydrate: A weighed amount of fresh tissue was homogenized with distilled water. The homogenate was filtered using a two-layered cheese cloth. The filtrate was then centrifuged at 10,000 rpm for 15 min. The supernatant was collected, and the volume was made up to 25 ml using distilled water. An aliquot of the sample was pipetted out and 4 ml Anthrone reagent added. It was then kept in a boiling water bath for 10 min. The tubes were cooled, and the absorbance was measured at 530 nm. The amount of total carbohydrate present was determined using the standard graph of glucose.
Estimation of Protein: A total protein present in the plant was estimated by Lowrey’s method. 1 gm powdered plant material was homogenized in 5 ml of 0.1 M PO4 buffer. The homogenate was filtered through double layered cheesecloth and centrifuged at 10,000 rpm for 10 min. The supernatant was collected, and the volume was made up to 1.5 ml by PO4 buffer. After that 1.5 ml of Bradford reagent was added and kept it for 5 min. The absorbance was recorded spectrophotometrically by using appropriate blank at 595 nm. The protein content was calculated from the standard graph of BSA or Bovine Serum Albumin.
TABLE 1: PHYTOCHEMICALS OF CASSIA FISTULA
| Test | Test method | Test Result |
| Flavonoid | Shinoda test | + |
| Glycoside | Killer-Killiani Test | + |
| Alkaloid | Mayer’s test | + |
| Tannin | Ferric chloride | + |
| Saponin | Foam test | + |
| Anthraquinone | + | |
| Steroids | Libermann-Burchard Test | + |
| Terpenoids | Libermann-Burchard Test | + |
| Reducing sugar | Fehling’s test | + |
GC-MS Analysis: GC-MS Gas chromatography-mass spectrometry (GC-MS) is a method that combines the features of gas-liquid chromatography and mass spectrometry to identify different Substances within a test sample.
The primary goal of instrument analysis is to quantify an amount of substance. This is done by comparing the relative concentrations among the atomic masses in the generated spectrum. Two kinds of analysis are possible, comparative and original. Comparative analysis essentially compares the given spectrum to a spectrum library to see if its characteristics are present for some sample in the library. This is best performed by a computer because there is a myriad of visual distortions that can take place due to variations in scale. Computers can also simultaneously correlate more data (such as the retention times identified by GC), to more accurately relate certain data.
Another method of analysis measures the peaks about one another. In this method, the tallest peak is assigned 100% of the value, and the other peaks being assigned proportionate values. All values above 3% are assigned. The parent peak normally indicates the total mass of the unknown compound. The value of this parent peak can be used to fit with a chemical formula containing the various elements which are believed to be in the compound. The isotope pattern in the spectrum, which is unique for elements that have many isotopes, can also be used to identify the various elements present. Once a chemical formula has been matched to the spectrum, the molecular structure and bonding can be identified and must be consistent with the characteristics recorded by GC-MS.
Typically, this identification is done automatically by programs which come with the instrument, given a list of the elements which could be present in the sample.
Preparation of Extract: 2 μl of the ethanolic extract of Cassia fistula was employed for GC/MS analysis.
Instruments and Chromatographic Conditions: GC-MS analysis was carried out on a GC-MS analysis was carried out on a GC Clarus 500 Perkin Elmer system comprising an AOC-20i autosampler and Gas chromatograph interfaced to a mass spectrometer (GC-MS) instrument employing the following conditions:
GC Programme:
- Column: Elite-5MS (5% Diphenyl / 95% Dimethyl poly siloxane), 30 × 0.25mm × 0.25 mm df
- Equipment: GC Clarus 500 Perkin Elmer
- Carrier gas: 1 ml per min, Split: 10:1
- Detector: Mass detector Turbo mass gold-Perkin Elmer
- Software: Turbo mass 5.2
- Sample injected: 2 ml
Oven Temperature Programme:
- 110 °C -2 min hold
- Up to 200 °C at the rate of 10 °C/min-No hold
- Up to 280 °C at the rate of 5 °C / min-9 min hold
- Injector temperature 250 °C
- Total GC running time 36 min
MS Programme:
- Library used NIST Version-Year 2005
- Inlet line temperature 200 °C
- Source temperature 200 °C
- Electron energy: 70 eV
- Mass scan (m/z): 45-450
- Solvent Delay: 0-2 min
- Total MS running time: 36 min
RESULTS:
Extraction: The phytochemicals present in the plant material was extracted by the distillation method using soxhlet apparatus. The solvent, ethanol was used for the separation of the chemical component.
Phytochemical Analysis: Standard phytochemical screening for flavonoid (Ferric chloride test), glycosides (Fehling’s test), alkaloids (Mayer’s test), tannin (Ferric chloride test), saponins (foam test) were done 8. The qualitative phytochemical investigations of Cassia fistula Linn. extract showed the presence of steroids, flavonoids, saponins, alkaloids, and tannin in the ethanol extracts.
Results showed the moisture content of the plant was found to be 90%, while the least content was found to be phenol which was only about 0.002 mg/g fresh tissue. The carbohydrate content is 8.35mg/g and phenol, protein; content is 0.0024, 1.94, mg/g respectively. Biochemical parameters such as protein, carbohydrate, phenol were analyzed, and the results were given.
TABLE 2: QUANTITATIVE ANALYSIS OF CASSIA FISTULA
| Phytochemical compounds | Amount present |
| Carbohydrate | 83.5mg/gm L |
| Protein | 1.94mg/gm L |
| Moisture content | 90% |
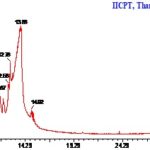
Compounds Identified: Cassia fistula: GC-MS chromatogram analysis of the ethanolic extract of Cassia fistula showed sixteen peaks which indicate the presence of sixteen phytochemical constituents. On comparison of the mass spectra of the constituents with the help of Dr. Duke’s Phytochemical and Ethanol botanical databases, the sixteen phyto compounds were characterized and identified. The various phytochemicals which contribute to the medicinal activities of the plant were shown. The mass spectra of all the phytochemicals identified in the whole plant ethanolic extract of Cassia fistula were shown.
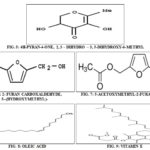
Of the sixteen compounds identified, the most prevailing compounds were 4 H-Pyran-4-one, 2,3-dihydro 3, 5 dihydroxy 6 methyl, 2 Furan carboxaldehyde, 5-(hydroxymethyl)-, 5-Acetoxy-methyl-2-furaldehyde, Oleic Acid, Cholesta-4,6-dien-3-ol, (3b’)-, vitamin E, b’-Sitosterol, Cholest-5-en-3-ol, 24-propylidene-, (3b’)-. Among the compounds, four compounds were reported to have anti-microbial property Butanoic acid, 2-methyl-, 2-methylpropyl ester, Pentanoic acid, 1,1-dimethylethyl ester, Valeric acid, 4-tridecyl ester, 2,4;3,5-Dimethylene-l-iditol of the sample.
TABLE 3: COMPONENTS IDENTIFIED IN THE PLANT SAMPLE-CASSIA FISTULA [GC-MS STUDY]
| S. no. | RT | Name of the compound | Molecular formula | MW | Peak Area % |
| 1 | 3.07 | Thymine | C5H6N2O2 | 126 | 1.16 |
| 2 | 3.65 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 0.49 |
| 3 | 4.12 | Butanoic acid, 2-methyl-, 2-methylpropyl ester | C9H18O2 | 158 | 0.77 |
| 4 | 4.68 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | C6H6O3 | 126 | 5.28 |
| 5 | 7.39 | Pentanoic acid, 1,1-dimethylethyl ester | C9H18O2 | 158 | 1.28 |
| 6 | 7.97 | 5-Acetoxymethyl-2-furaldehyde | C8H8O4 | 168 | 1.80 |
| 7 | 10.59 | Valeric acid, 4-tridecyl ester | C18H36O2 | 284 | 0.97 |
| 8 | 11.67 | 2,4;3,5-Dimethylene-l-iditol | C8H14O6 | 206 | 12.76 |
| 9 | 12.76 | n-Hexadecanoic acid | C16H32O2 | 256 | 7.35 |
| 10 | 13.88 | Myo-Inositol, 4-C-methyl- | C7H14O6 | 194 | 64.82 |
| 11 | 14.92 | Oleic Acid | C18H34O2 | 282 | 1.50 |
| 12 | 23.94 | Squalene | C30H50 | 410 | 0.08 |
| 13 | 27.21 | Cholesta-4,6-dien-3-ol, (3á)- | C27H44O | 384 | 0.27 |
| 14 | 28.15 | Vitamin E | C29H50O2 | 430 | 0.27 |
| 15 | 31.19 | á-Sitosterol | C29H50O | 414 | 0.75 |
| 16 | 31.53 | Cholest-5-en-3-ol, 24-propylidene-, (3á)- | C30H50O | 426 | 0.47 |
FIG. 4: GC-MS CHROMATOGRAM - CASSIA FISTULA
Structure of Compounds having Anti-Inflammatory Property:
DISCUSSION: In spite of tremendous development in the field of synthetic drugs during the recent era, they are found to have some or other side effects, whereas plants still hold their unique place, by way of having no side effects. Therefore, a systematic approach should be made to find out the efficacy of plants against inflammation to exploit them as herbal anti-inflammatory agents. The potential effect of the ethanolic extract of Cassia fistula was investigated.
Recent studies suggest that the inflammatory tissue damages are due to the liberation of reactive oxygen species from phagocytes invading the inflammation Sites 9-10-11. In addition to this, nitric oxide is also implicated in inflammation, cancer, and other pathological conditions 12. Interactions between superoxide and nitric oxide regulate the vascular tone or inflammation.
Cassia fistula contains alkaloids, tannins, flavonoids, terpenes, sugars, and glucosides. Flavonoids have been shown to possess various biological properties related to the antioxidant, antinociceptive and anti-inflammatory mechanisms by targeting reactive oxygen species and prostaglandins which are involved in the late phase of acute inflammation and pain perception.
Using the results obtained from GC-MS, we found the presence of compounds which are active against inflammation.
CONCLUSION: In the present study, we carried out several tests to evaluate the anti-inflammatory activity of Cassia fistula. Qualitative and Quantitative phytochemical analysis was done. From the results, we found that our plant species contains many effective compounds like flavonoids, alkaloids, tannin, anthraquinone, etc. Further, we analyzed our samples using gas chromatography and mass spectrometry (GC-MS).
Based on the GC-MS results obtained we conclude that Cassia fistula has anti-inflammatory activity has more.
Further, we planned to do in-vitro studies using animals. There are certain problems associated with the use of animals in experimental pharmacological research such as ethical issues and the lack of rationale for their use.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Verpoorte R, van der Heijden R, Ten Hoopen HJG and Memelink J: Metabolic engineering of plant secondary metabolite pathways for the production of new chemicals. Biotechnol Lett 1999, 21: 467-479.
- Joshi KP, Chavan D and Patwardhan WB: Molecular markers in herbal drug technology. Cwr Sci 2004; 87: 159-165.
- Chopra NC, Nayar SL and Chopra IC: Glossary of Indian medicinal plants, CSIR, New Delhi 1956: 81.
- Batna PA and Balaraman R: Faculty of Technology and Engineering; Phytomedicine 2005; 12(4): 264-270.
- Anonymous. New medical dictionary. 2nd ed. Oxford and IBH Publishing Co. Pvt.Ltd. New Delhi 2005.
- Tripathi KD: Essentials of medical pharmacology. 6th ed. Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi 2008.
- Kokashi CJ, Kokashi RJ and Sharma M: Fluorescence of powdered vegetable drugs in ultra-violet radiation, J Am Pharm Assoc 1958; 47: 715-717.
- Farnsworth NR: Biological and phytochemical screening of plants. J Pharm Sci 1966; 55: 225-276.
- Harbone JB: Phenolic glycosides and their natural distribution in the biochemistry of phenolic compounds, Academic Press, New York, London 1973; 152-162.
- Thomas-Barberan FA and Msonthi JD: Hostettmann K. Phytochemistry 1988; 27(3): 753-755.
- Pratt RT and Chase ER: Fluorescence powder vegetable drugs in particular to development system of identification, J Am Pharm Assoc 1949; 38: 324-331.
- Huang SS, Chiu CS and Chen HJ: Antinociceptive activities and the mechanisms of anti-inflammation of asiatic acid in mice. Evidence-Based Complementary and Alternative Medicine pages 2011.
How to cite this article:
Anitha J and Miruthula S: Anti-inflammatory and phytochemicals analysis of Cassia fistula Linn. Fruit pulp extracts. Int J Pharmacognosy 2014; 1(3): 207-15. doi: 10.13040/IJPSR.0975-8232.1(3).207-15.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
8
207-15
693
3041
English
IJP
J. Anitha * and S. Miruthula
Department of Biotechnology, Arunai Engineering College Thiruvannamalai, Tamil Nadu, India.
mvragav444@yahoo.com
02 December 2013
13 February 2014
28 February 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(3).207-15
01 March 2014