ANTI-CANCER AGENTS DERIVED FROM PLANT AND DIETARY SOURCES: A REVIEW
HTML Full TextANTI-CANCER AGENTS DERIVED FROM PLANT AND DIETARY SOURCES: A REVIEW
Md. Uzzal Haque *, Nowrin Ferdiousi and Sadiur Rahman Sajon
Department of Pharmacy, Faculty of Biological Science and Technology, Jessore University of Science and Technology, Jessore - 7408, Bangladesh.
ABSTRACT: Cancer is a disease of deregulated cellular behavior. Acquisition of oncogenic attributes, loss of tumor suppressive functions, evasion of physiological tissue architecture and interactions with the cellular microenvironment enable malignant cells to escape the mechanisms of normal cellular homeostasis in an organism. Cancer cells are therefore able to sustain unlimited proliferation, to thrive under conditions that preclude normal cell survival, and to spread to distant sites through the process of metastasis. Natural products are important sources of new anticancer drugs, new drug leads and new chemical entities. The plant-based drug discovery resulted mainly in the development of anticancer agents including plants (vincristine, vinblastine, paclitaxel, etoposide, camptothecin, topotecan, and irinotecan). Beside this there are numerous agents identified from fruits and vegetables can use in anticancer therapy. The agents include curcumin (turmeric), resveratrol (red grapes, peanuts and berries), genistein (soybean), diallyl sulfide (allium), S-allyl cysteine (allium), allicin (garlic), lycopene (tomato), capsaicin (red chilli), diosgenin (fenugreek), 6-gingerol (ginger), ellagic acid (pomegranate), ursolic acid (apple, pears, prunes), silymarin (milk thistle), anethol (anise, camphor, and fennel), catechins (green tea), eugenol (cloves), indole-3-carbinol (cruciferous vegetables), Limonene (citrus fruits), beta carotene (carrots), and dietary fiber. In this review active principle derived from natural products are offering an excellent opportunity to evaluate not only new chemical classes of anticancer agents but also novel lead compound and potentially relevant mechanisms of action.
| Keywords: |
Anti-cancer activity, Vincristine, Vinblastine, Vegetables, Fruits
INTRODUCTION: In the most general terms, cancer refers to cells that grow out-of-control and invade other tissues. Cells become cancerous due to the accumulation of defects, or mutations, in their DNA. Certain inherited genetic defects (for example, BRCA1 and BRCA2 mutations) and infections can increase the risk of cancer.
Environmental factors (for example, air pollution) and poor lifestyle choices such as smoking and heavy alcohol use can also damage DNA and lead to cancer. Most of the time, cells can detect and repair DNA damage. If a cell is severely damaged and cannot repair itself, it undergoes so-called programmed cell death or apoptosis. Cancer occurs when damaged cells grow, divide, and spread abnormally instead of self-destructing as they should.
The International Agency for Research on Cancer showed in research that 184 countries of the world had 14.1 million new cancer cases, 8.2 million cancer deaths, and 32.6 million people living with cancer (within 5 years of diagnosis) in 2012 worldwide 1. It is projected that there will be 26 million new cancer cases and 17 million cancer deaths per year by 2030 2. The main processes of cancer treatment in humans are surgery, radiation, and drugs (chemotherapeutic agents). Cancer chemotherapeutic agents can often provide temporary relief of symptoms, give the patient more time and rarely cures. In recent years, a lot of research has been conducted to the synthesis of potential anticancer drugs. Many hundreds of chemical compounds of a known class of anticarcinogenic agent have been synthesized but have lots of side effects. A successful anticancer drug should kill or inactivate cancer cells without causing excessive damage to normal cells 3, 4. Therefore there is a constant necessity for developing new, useful and relatively anticancer drugs 5. Chemical compounds derived from plants have been used to treat human diseases from the dawn of human civilization. Natural products have caught increasing attention over the past few years for their potential as a novel cancer preventive and therapeutic agents 6, 7.
In parallel, there is increasing evidence for the potential ability of plant-derived compounds as inhibitors of various stages of tumor cell formation and associated inflammatory processes, indicating the importance of these products in cancer prevention and therapy.
The search for anti-cancer agents from plant sources started in earnest in the 1950s with the discovery and development of the vinca alkaloids, vinblastine and vincristine, and the isolation of the cytotoxic podophyllotoxins. This led to the discovery of many novel chemotypes showing a range of cytotoxic activities 8 including the taxanes and camptothecins, but their development into clinically active agents spanned a period of some 30 years, from the early 1960s to the 1990s. This plant collection program was terminated in 1982, but the development of new screening technologies led to the revival of collections of plants and other organisms in 1986, with a focus on the tropical and sub-tropical regions of the world. It is interesting to note, however, that no new plant-derived clinical anti-cancer agents have, as yet, reached the stage of general use, but some agents are in preclinical development.
Plant-Based Anticancer Drugs:
1. Vinka Alkaloid: Vinka alkaloids are anti-mitotic and anti-microtubule alkaloid agents initially derived from the periwinkle plant Catharanthus roseus (basionym Vinca rosea) and other vinca plants. Vinca alkaloids are used in chemotherapy for cancer. They are a class of cell cycle–specific cytotoxic drugs that work by inhibiting the ability of cancer cells to divide: Acting upon tubulin; they prevent it from forming into microtubules, a necessary component for cellular division. Vinka alkaloid includes- Vinblastine (VLB) and Vincristine (VCR), Vinorelbine (VRLB) and Vindesine (VDS) are obtained from the Catharanthus roseus G. Don. (Apocynaceae).
Vincristine: Vincristine knew as leurocristine, sometimes abbreviated "VCR," is a vinca alkaloid from the Catharanthus roseus (Madagascar periwinkle), formerly Vinca rosea and hence its name. Vincristine’s blockage of microtubule formation is especially powerful. The reason for this comes from the fact that tubulin protein is dynamic. Its long chain of building blocks is always growing in some places and breaking in others. The less contiguous parts of a tubulin molecule have pieces only two building blocks long, called dimers. Vincristine has a high affinity for tubulin dimers, and the reaction between vincristine and the dimers is rapidly reversible. That means a vincristine molecule will attach to a dimer at one site, break off, and then reattach at another site. This keeps two sites per dimer “poisoned” and unable to reassemble into the protein. So vincristine’s ability to destabilize tubulin is especially good.
Vincristine: Disruption of the microtubules arrests mitosis in metaphase. Therefore, the vinca alkaloids affect all rapidly dividing cell types including cancer cells, but also those of intestinal epithelium and bone marrow. The main side-effects of vincristine are peripheral neuropathy, hyponatremia, constipation, and hair loss.
Vincristine is delivered via intravenous infusion for use in various types of chemotherapy regimens. Its main uses are in non-Hodgkin's lymphoma as part of the chemotherapy regimen CHOP, Hodgkin's lymphoma as part of MOPP, COPP, BEACOPP, or the less popular Stanford V chemotherapy regimen, in acute lymphoblastic leukemia, and in treatment for nephroblastoma (Wilms tumor, a kidney tumor most common in young children). It is used in combination with prednisone to treat childhood leukemia.
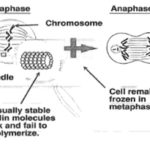
Vinblastine: Vinblastine (VLB) is a naturally occurring active compound. Vinblastine sulfate is the salt of an alkaloid extracted from Vinca rosea Linn., a common flowering herb known as the periwinkle (more appropriately known as Catharanthus roseus G. Don). Previously, the generic name was vinca leukoblastine, abbreviated VLB. It is a stathmo kinetic oncolytic agent. When treated in vitro with this preparation, growing cells are arrested in metaphase. Vinblastine should not be given intramuscularly, subcutaneously or intra thecally.
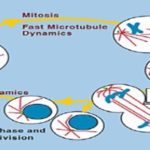
Microtubule disruptive drugs like vinblastine, colcemid, nocodazole have been reported to act by two mechanisms. At very low concentrations they suppress microtubule dynamics, and at higher concentrations they reduce microtubule polymer mass. Vinblastine includes adverse effects are nausea and vomiting which usually lasts less than 24 hours, stomach pain, constipation, diarrhea, jaw pain, headache, or another ache, thinned or brittle hair, exposed areas of the skin may become easily sunburned. Vinblastine is an anti-cancer medication prescribed in various cancers such as Hodgkin's lymphoma, non-Hodgkin's lymphoma, breast cancer, testicular cancer, mycosis fungoides, Kaposi's sarcoma related to acquired immunodeficiency syndrome (AIDS), Letterer-Siwe disease. Vinblastine is also used to treat non-small cell lung cancer, bladder cancer, head and neck cancer, cervical cancer, idiopathic thrombocytopenia purpura, and autoimmune hemolytic anemia. Vinblastine sulphate is contraindicated in patients who are leucopenia. It should not be used in the presence of bacterial infection. Such infections should be brought under control.
FIG.2: MECHANISM OF VINBLASTINE 2
Vinorelbine: Vinorelbine is the first 5´NOR semi-synthetic vinca alkaloid. It is obtained from alkaloids from the rosy periwinkle, Catharanthus roseus. The antitumor activity of vinorelbine is thought to be due primarily to inhibition of mitosis at metaphase through its interaction with tubulin. Vinorelbine binds to the microtubular proteins of the mitotic spindle, leading to crystallization of the microtubule and mitotic arrest or cell death. Like other vinca alkaloids, vinorelbine may also interfere with:
Vinorelbine Amino acid, cyclic AMP, and glutathione metabolism, 2) calmodulin dependent Ca2+-transport ATPase activity, 3) cellular respiration, and 4) nucleic acid and lipid biosynthesis. Adverse effects of vinorelbine are Lowered resistance to infection, bruising or bleeding, anemia, constipation, diarrhea, nausea, numbness or tingling in hands or feet (peripheral neuropathy), tiredness and a general feeling of weakness (asthenia), inflammation of the vein into which it was injected (phlebitis). Seldom severe hyponatremia is seen. Less common effects are hair loss and allergic reaction. Vinorelbine is approved for the treatment of non-small cell lung cancer and metastatic breast cancer. It is also active in rhabdomyosarcoma. Administration of Vinorelbine is contraindicated in patients with pretreatment granulocyte counts <1,000 cells/ mm3.
2. Epipodophyllotoxin: The most studied lignan, podophyllotoxin, and its semisynthetic derivatives (etoposide, teniposide, etoposide phosphate), are particularly impressive at a curative level due to their cytotoxic properties. These semi-synthetic derivatives are used in chemotherapy of lung cancer. Podophyllin, an ethanolic extract of Podophyllum peltatum L. or P. emodi Wall (syn. P. hexandnum Royle), is a good source of the aryltetralin-type lignan, podophyllotoxin.
Etoposide: Etoposide phosphate is an anticancer agent, which belongs to the drug type topoisomerase inhibitor. Etoposide forms a ternary complex with DNA and the topoisomerase II enzyme (which aids in DNA unwinding), prevent re-ligation of the DNA strands, and by doing so causes DNA strands to break.
Adverse effects of etoposide include low blood pressure, hair loss, pain and or burning at the IV site, constipation or diarrhea, metallic food taste, Bone marrow suppression, leading to decreased white blood cell counts (leading to increased susceptibility to infections), low red blood cell counts (anemia), low platelet counts (leading to easy bruising and bleeding), nausea and vomiting, allergic-type reactions, rash, fever often occurring shortly after IV administration and not due to infection, mouth sores, acute myeloid leukemia (which ironically can be treated with etoposide itself).
Etoposide is used as a form of chemotherapy for cancers such as Kaposi’s sarcoma, Ewing's sarcoma, lung cancer, testicular cancer, lymphoma, nonlymphocytic leukemia, and glioblastoma multiforme. It is also sometimes used in a conditioning regimen before a bone marrow or blood stem cell transplant. Etoposide contraindicated In Hypersensitivity, pregnancy, lactation.
3. Taxanes: The prototype taxane is the natural product paclitaxel, known initially as Taxol and first derived from the bark of the Pacific Yew tree. Docetaxel is a semi-synthetic analog of paclitaxel. Taxanes enhance the stability of microtubules, preventing the separation of chromosomes during anaphase.
Paclitaxel: A cyclohexane isolated from the bark of the Pacific yew tree, Taxus brevifolia. It stabilizes microtubules in their polymerized form leading to cell death.
Paclitaxel interferes with the normal function of microtubule growth. Paclitaxel binds to the β subunit of tubulin. Tubulin is the "building block" of microtubules, and the binding of paclitaxel locks these building blocks in place. The resulting micro-tubule/paclitaxel complex cannot disassemble.
This adversely affects cell function because the shortening and lengthening of microtubules (termed dynamic instability) are necessary for their function as a transportation highway for the cell. Further research has indicated that paclitaxel induces programmed cell death (apoptosis) in cancer cells by binding to an apoptosis stopping protein called Bcl-2 (B-cell leukemia 2) and thus arresting its function.
FMITOSIS BLOCKED BY PACLITAXEL 3
Common side effects include nausea and vomiting, loss of appetite, change in taste, thinned or brittle hair, pain in thejoints of the arms or legs lasting two to three days, changes in the color of the nails, and tingling in the hands or toes. More serious side effects such as unusual bruising or bleeding, pain/redness/swelling at the injection site, change in normal bowel habits for more than two days, fever, chills, cough, sore throat, difficulty swallowing, dizziness, shortness of breath, severe exhaustion, skin rash, facial flushing, female infertility by ovarian damage and chest pain canalso occur.
A number of these side effects are associated with the excipient used, Cremophor EL, a polyoxyethylatedcastor oil. Allergies to drugs such as cyclosporine, teniposideand drugs containing polyoxyethylated castor oil may indicate they increased the risk of adverse reactions to paclitaxel. Paclitaxel is approved for ovarian, breast and lung cancers and Kaposi's sarcoma. Paclitaxel should be available for the treatment of advanced breast cancer after the failure of anthracyclic chemotherapy, but that its first-lineuse should be limited to clinical trials.
Paclitaxel is contraindicated in patients with severe hypersensitivity to paclitaxel or any excipient, especially macrogolglycerolricinoleate (polyoxy-ethylated castor oil). Paclitaxel is contraindicated during pregnancy and lactation, and should not be used in patients with baseline neutrophils < 1,500/mm³ (<1,000/mm³ for KS patients). In KS, paclitaxel is also contraindicated in patients with concurrent, severe and uncontrolled infection.
TABLE 1: PLANT BASED ANTICANCER AGENTS IN CLINICAL PRACTICE.
| Compounds | Uses | Status |
| Vincristine | Leukemia, lymphoma, breast, lung, pediatric solid cancers and others | Phase III/IV |
| Vinblastine | Breast, lymphoma, germ-cell and renal cancer | Phase III/IV |
| Vinorelbine | Lymphoma, tumors, Lung
cancer |
Phase I-III |
| Paclitaxel | Ovary, breast, lung, bladder and head and neck cancer | Phase III/IV |
| Docetaxel | Breast and lung cancer | Phase III |
| Topotecan | Ovarian, lung and pediatric cancer | Phase II/III |
Docetaxel: Docetaxel (as generic or under the trade name Taxotere) is a well-established anti-mitotic chemotherapy medication (that is, it interferes with cell division). The cytotoxic activity of docetaxel is exerted by promoting and stabilizing microtubule assembly while preventing physiological microtubule depolymerization/disassembly in the absence of GTP. This leads to a significant decrease in free tubulin, needed for microtubule formation and results in inhibition of mitotic cell division between metaphase and anaphase, preventing further cancer cell progeny. Because microtubules do not disassemble in the presence of docetaxel, they accumulate inside the cell and cause initiation of apoptosis.
Apoptosis is also encouraged by the blocking of apoptosis-blocking bcl-2oncoprotein. Both in vitro and in-vivo analysis show the antineoplastic activity of docetaxel to be effective against a wide range of known cancer cells, cooperate with other antineoplastic agents activity, and have greater cytotoxicity than paclitaxel, possibly due to its more rapid intracellular uptake. This includes tumor cells as well as hair follicles, bone marrow, and other germ cells. For this reason, common chemotherapy side effects such as alopecia occur; sometimes this can be permanent.
Hematological adverse effects include Neutropenia (95.5%), Anemia (90.4%), febrile neutropenia (11.0%) and thrombocytopenia (8.0%).The main use of docetaxel in the treatment of a variety of cancers after the failure of anthracycline-basedchemotherapy 4. Marketing of docetaxel as Taxotere is mainly towards the treatment of breast, prostate, and other non-small cell cancers5. Clinical data has shown docetaxel to have cytotoxic activity against breast, colorectal, lung, ovarian, prostate, liver, renal, gastric, head and neck cancers, and melanoma. Docetaxel is contraindicated for use with patients with; a baseline neutrophil counts less than 1500 cells/μL, a history of severe hypersensitivity reactions to docetaxel or polysorbate80, severe liver impairment, and pregnant or breastfeeding women.
4. Camptothecins: Camptothecin (CPT) is a cytotoxic quinoline alkaloid which inhibits the DNA enzyme topoisomerase I (topo I). It was discovered in 1966 by M. E. Wall and M. C. Wani in the systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata (Camptotheca, Happy tree), a tree native to China used as a cancer treatment in Traditional Chinese Medicine.
Topotecan: Topotecan (trade name Hycamtin) is a chemotherapeutic agent that is a topoisomerase inhibitor. It is a water-soluble derivative of camptothecin. It's brand name is Hycamptamine, Hycamptin, Hycamtin. Topotecan has the same mechanism of action as irinotecan and is believed to exert its cytotoxic effects during the S phase of DNA synthesis. Topoisomerase I relieves torsional strain in DNA by inducing reversible single-strand breaks. Topotecan binds to the topoisomerase I-DNA complex and prevents religation of these single-strand breaks. This ternary complex interferes with the moving replication fork, which leads to the induction of replication arrest and lethal double-stranded breaks in DNA.
Topotecan: As mammalian cells cannot efficiently repair these double-strand breaks, the formation of this ternary complex eventually leads to apoptosis (programmed cell death). Side effects include Myelosuppression, Diarrhea, Low blood counts, Susceptibility to infection. Topotecan is used to treat patients with metastatic cancer (cancer that has already spread) of the ovaries after other treatments have failed. This medicine is also used to treat a certain type of lung cancer called small cell lung cancer. It is also used in combination with cisplatin to treat cancer of the cervix which cannot be treated with surgery or radiation therapy.
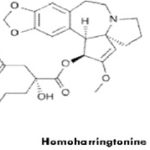
5. Cephalotaxanes: Cephalotaxus [C. harringtonia and C. fortuni]-source of harringtonine, it is a promising new anti-cancer alkaloid.
Homoharringtonine:Omacetaxinemepesuccinate (INN, or homoharringtonine, trade name Omapro) is an alkaloid from Cephalotaxus harringtonia that is investigated for potential use as a drug against hematological cancers. It is being developed by Chem Genex and is on fast track approval schedule in the United States. Omacetaxine has been granted orphan drug status in the U.S. and Europe.
Omacetaxine induces apoptosis by inhibition of protein synthesis, particularly Mcl-1. It has a different point of action than tyrosine kinase inhibitors like imatinib and has potential therapeutic advantages for patients who have developed resistance to tyrosine kinase inhibitor therapy. Nausea and vomiting, diarrhea, and fever and chills were the most common side effects. Serious reversible cardiovascular toxicity, which occurred in three patients, included symptomatic hypotension in two and short runs of ventricular tachycardia in one. Use in sarcoma and breast cancer as well as in ovarian and endometrial carcinoma, in solid tumors, in myeloid leukemia, myelodysplastic syndrome, acute promyelocytic leukemia, and, most important, chronic myeloid leukemia (CML).
6. Flavones: In recent years, flavonoids and their synthetic analogs have been intensely investigated in the treatment of ovarian, breast, cervical, pancreatic, and prostate cancer.
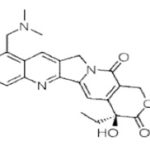
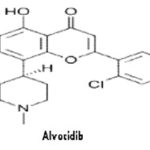
Flavopiridol (Alvocidib): Rohitukine (C16H19O5N), a chromane alkaloid, was first reported from Amoora rohituka (Roxb.) Wight & Arn. And then from Dysoxylum binectariferum Hook both from the family Meliaceae. Alvocidib (also known as Flavopiridol HMR-1275) is a cyclin-dependent kinase inhibitor under clinical development for the treatment of chronic lymphocytic leukemia. It has also been studied for the treatment of arthritis. A phase I/II study of Flavopiridol to treat relapsed mantle cell lymphoma or diffuse large B-cell lymphoma has been completed.
Alvocidib inhibits cyclin-dependent kinases, arresting cell division and causing apoptosis in non-small lung cancer cells. Main side effects were secretory diarrhea and a pro-inflammatory syndrome associated with hypotension and investigated for use/treatment in esophageal cancer, leukemia (lymphoid), lung cancer and liver cancer.
Herbs and Anti-Oxidants that Fight with Cancer:
Garlic: The National Cancer Institute (affiliated to the NIH) recognizes garlic to have potential anticancer properties. The sulphydryl compounds in garlic can block the formation of cancer-causing substances. Several population studies have shown an association between increased garlic consumption and reduced risk of cancers of the stomach, colon, esophagus, pancreas, and also breast cancer. A study has found that garlic intake of 10 g per day could reduce the risk of prostate cancer by 50 percent.
Ginger: Some pungent substances present in ginger rhizome have anti-oxidant and anti-inflammatory activities. The anti-cancer properties of ginger are attributed to phenolic materials such as 6-gingerol and 6-paradol and other constituents such as shogaols and zingerone. A study published in the journal Biochemical and Biophysical Research Communications reported that 6-gingerol could reduce the viability of gastric cancer cells and limit the spread of cancer.
Turmeric: Although turmeric is promoted mainly as an anti-inflammatory herbal remedy, some scientists believe that the anti-oxidant curcumin present in turmeric may prevent or slow the growth of many cancers including tumor of esophagus, stomach and intestine, breast cancer and also skin cancer in experimental animals. However, clinical research is needed to determine its efficacy in cancer prevention and treatment in human beings. But, the laboratory studies have confirmed the curcumin interferes with several molecular pathways involved in cancer development, growth and spread. Further, a study found that ethanolic extract of turmeric produces remarkable symptomatic relief in patients with external cancerous lesions. There was a reduction in smell in 90 percent of cases and reduction in itching in almost all cases.
Green Tea: Polyphenols in green tea and sometimes black tea, help kill cancerous cells and stop their progression. Mayo Clinic studies have revealed that a substance called epigallocatechin gallate (EGCG) in green tea reduces the number of leukemia cells in patients with CLL (Chronic lymphocytic leukemia), a form of blood cancer. Similarly, another study found that women who drank powdered green tea were less likely to develop bladder cancer. Again, men who drank the greenest tea were 37 percent less likely to develop pancreatic cancer. A large Chinese clinical study found.
Cilantro: Cilantro or, more commonly, coriander is another potent herb that has anti-cancer properties. The prevalent anti-oxidants in cilantro are beta-carotene, quercetin, and rutin. This herb, generally used in chelation therapy for people suffering from lead poisoning, helps remove free radicals by getting rid of the heavy metals in your body.
Basil: Basil is well known for its medicinal value. Apart from having anti-inflammatory, blood pressure lowering, and nervous system stimulating properties, this popular herb has been found to have the chemoprotective potential for colon cancer. A study found that basil played a significant role in reducing colon tumors in experimental animals. However, no human clinical trials have been conducted to confirm this experiment.
Survival of Plants used as Anticancer Agents: The World Health Organization estimates that approximately 80% of the world’s inhabitants rely on traditional medicine for their primary health care 6. The National Cancer Institute collected about 35,000 plant samples from 20 countries and has screened around 114,000 extracts for anticancer activity. From this screening two or three most important anti-cancer compounds available today, namelytaxol and camptothecin. Various types of anti-cancer plant are zedoary (Curcuma zedoaria), marijuana (Cannabis sativa), Indian trumpet (Oroxylum Indicum), celandine (Chelidonium majus), yew (Taxus baccata), turmeric (Curcuma longa), rodent tuber (Typhoniumflagelliforme), god’s crown (Phaleriama crocarpa), madagaskar periwinkle (Catharanthus rosens), artocarpus integer (Selaginella corymbosa), bamboo grass (Loathatreum gràcies), handsome (Taraxacum mongolicum), fruit makasar (Brucca javanica), garlic (Allium sativum), echo china (Smilax china), sunflower (Helianthus annus), leunca (Solanum nigrum), job’s tears (Coix lachryma-Jobi), bamboo rope (Asparagus cochinchinensis), acanthopanax root bark (Acanthopanax gracilistylus), licorice (Glycyrrhiza glabra) etc. A brief description of medicinal plants in Asian origin used for the prevention and treatment of cancer is given below. The review provides information on the active anticancer components of the plants.
Dietary Source of Anti-Cancer Agents: Natural dietary agents including fruits, vegetables, and spices have drawn a great deal of attention from both the scientific community and the general public owing to their demonstrated ability to suppress cancers. Recent studies suggest that the consumption of food rich in fruits, vegetables, and spices have a lower incidence of cancers (stomach, esophagus, lung, oral cavity and pharynx, endometrium, pancreas, and colon).
TABLE 2: PLANTS USED AS ANTI-CANCER
| S. no. | Plant species | Family | Plant part |
| 1 | Salvia officinalis | Labiatae | Leaves 7 |
| 2 | Viscum album | Loranthaceae | Leaves 8 |
| 3 | Combretum caffrum | Combretaceae | Bark 9 |
| 4 | Melaleuca alternifolia | Myrtaceae | Leaves 10 |
| 5 | Lavandula angustifolia | Labiatae | Leaves 10 |
| 6 | Aglaia foveolata | Meliaceae | Fruit 11 |
| 7 | Maytenus serrata | Celastraceae | Seed 12 |
| 8 | Tabebuia impetiginosa | Bignoniaceae | Stem bark and trunk wood 13, 14 |
| 9 | Tabebuia rosea | Bignoniaceae | Stem bark and trunk wood 13, 14 |
| 10 | Tabebuia serratifolia | Bignoniaceae | Stem bark and trunk wood 13, 14 |
| 11. | Dipteryx odorata | Fabaceae | Seed 15 |
| 12 | Thapsia garganica | Apiaceae | Fruit 16 |
| 13 | Indigo feratinctoria | Leguminosae | Aerial part 17 |
| 14 | Matricaria chamomilla | Asteraceae | Flower 18 |
| 15 | Erythroxylum pervillei | Erythroxylaceae | Root 19 |
| 16 | Broussonetia papyrifera | Urticaceae | Entire 20 |
| 17 | Cyclopia intermedia | Fabaceae | Leaves 21 |
| 18 | Scutellariae radix, Scutellariae indica | Labiatae | Root 23 |
| 19 | Physalis philadelphica | Solanaceae | Seed 23 |
| 20 | Dysoxylum binectariferum | Meliaceae | Stem bark 24 |
| 21 | Aristotelia chilensis | Elaeocarpaceae | Leaf and Stem 25 |
| 22 | Cyathostemma argentium | Annonaceae | Root 26 |
| 23 | Epimedium hunanense | Berberidaceae | Aerial parts 27 |
| 24 | Croton urucurama | Euphorbiacaeae | Bark 28 |
| 25 | Epilobium hirsutum | Onagraceae | Entire 29 |
| 26 | Pleione bulbocodioides | Orchidaceae | Tuber 30 |
| 27 | Cassia quinquangulata | Caesalpiniaceae | Root 31 |
| 28 | Begonia glabra | Begoniaceae | Entire 32 |
| 29 | Celastrus orbiculatus | Celastraceae | Entire 32 |
| 30 | Croton draco | Euphorbiacaeae | Aerial parts 33 |
| 31 | Smilax sieboldii | Liliaceae | Entire 33 |
| 32 | Ximenia Americana
Lymphoma (unspecified) |
Olacaceae | Root 33 |
TABLE 3: ANTICANCER AGENT DERIVED FROM DIETARY SOURCES
| S. no. | Botanical Name | Source | Compound |
| 1 | Carica papaya, Family- Caricaceae | Berries | β-Cryptoxanthin 34 |
| 2 | Glycyrrhiza glabra; Glycyrrhiza radix; Glycyrrhiza uralensis; Family: Leguminosae | Licorice root | Glycyrrhizin 35 |
| 3 | Cannabis sativa; Family: Cannabiaceae | Hemp | Cannabinol 36 |
| 4 | Rosamarinus officinalis; Family: Lamiaceae | Rosemary | Carnosol 37 |
| 5 | Glycine max; Family: Fabaceae | Soybeans | Genistein 38 |
| 6 | Prunus armeniaca; Family: Rosaceae | Apricots | Carotenoids 39 |
| 7 | Zingiber officinale; Family: Zingiberaceae | Tuber | Gingerol 40 |
| 8 | Lycopersicon esculentum; Family: Solanaceae | Tomato | Lycopene, Lutein, Kaempferol 40 |
| 9 | Piper nigram; Piper longum; Family: piperaceae | Black pepper | Purpurogallin; Piperine 41 |
| 10 | Ocimum sanctum; Family: Lamiaceae | Basil | Ursolic acid 42 |
| 11. | Betula alba; Family: Betulaceae | Birch tree | Betulinic acid 43 |
| 12 | Crocus sativus; Family: Iridaceae | Saffron | Carotenoids 44 |
| 13 | Silymarin marianum; Family: Asteraceae | Milk thistle | Silymarin 45 |
| 14 | Capsicum annum; capsicum frutescans; Family: Solanaceae | Red chilli | Capsaicinoids, Capsaicia 46 |
| 15 | Camelia sinensis; Family: Theaceae | Green and black teas | Catechin and flavins 47 |
| 16 | Vitis vinfera; Family: Vitaceae | Grapes | Resveratrol 48 |
| 17 | Daucuscarota sativa; Family: Apiaceae/ Umbelliferae | Carrot | β-Carotene 49 |
| 18 | Tabebuia avellanedae; Family: Bignoniaceae | Lapacha tree | Lapachone |
| 19 | Citrus Aurantium; Family: Rutaceae | Orange | Hesperidin 50 |
| 20 | Prunus dulcis, Family- Rosaceae | Almond | Morin 51, 52 |
| 21 | Aloe arborescens; Family: Asphodelaceae | Aloe vera | Emodin 53 |
| 22 | Opium poppy; Family: Papaveraceae | Poppy | Morphine and its analogs 54 |
| 23 | Cucurbita moschata; Family: Cucurbitaceae | Pumpkin | β-Carotene 54 |
| 24 | Azadirachata indica; Family: Meliaceae | Neem | Polyphenolics 55 |
Dietary derivatives consist of a wide variety of biologically active components that are responsible for the anti-cancer properties. Examples- are curcumin, genistein, resveratrol, diallyl sulfide, S-allyl cysteine, allicin, lycopene, capsaicin, diosgenin, gingerol, ellagic acid, ursolic acid, silymarin, anethol, catechins, eugenol, isoeugenol, dithiolthiones, isothiocyanates, indole-3-carbinol, isoflavones, saponins, phytosterols, inositol hexaphosphate, vitamin C, D-limonene, lutein, folic acid, beta carotene, selenium, vitamin E and flavonoids. Many of which have been used in traditional medicines for thousands of years. These dietary agents are believed to suppress the inflammatory processes that lead to transformation, hyperproliferation, and initiation of carcinogenesis. Their inhibitory influences may ultimately contain the final steps of carcinogenesis i.e. angiogenesis and metastasis.
CONCLUSION: Natural products are a prime source for the treatment of many forms of cancer, many of which are consumed daily with the diet. They provide significant protection against various cancers and many other diseases. The antioxidant medicinal plants and their products prevent the cancer and other diseases by protecting cells from damage. Thus, consuming a diet rich in antioxidant fruits, vegetables, herbs, etc. will provide health-protective effects. All the natural products discussed in this review exhibit anticancer activities. Natural products offer an excellent opportunity to evaluate not only new chemical classes of anticancer agents, but also novel and potentially relevant mechanisms of action.
ACKNOWLEDGEMENT: We are grateful to the authors/editors of all those articles has been reviewed and discussed.
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest between them.
REFERENCES:
- GLOBOCAN 2012, http://globocan.iarc.fr/Default.aspx
- Thun MJ, DeLancey JO, Center MM, Jemal A and Ward EM: The global burden of cancer: priorities for prevention. Carcinogenesis 2009; 31(1): 100-110.
- Kutluk T and Kars A: General Knowledge About Cancer. Ankara, Turkey cancer investigation and fight society publications 1998: 7-15.
- Kappor LD: “CRC Handbook of Ayurvedic Medicinal Plants, Boca Raton, Florida, CRC Press 1990: 416-417.
- Coseri S: Natural products and their analogs as efficient anticancer drugs. Mini-Reviews in Medicinal Chemistry 2009; 9(5): 560-571.
- Newman DJ: Natural products as leads to potential drugs: an old process or the new hope for drug discovery? Journal of Medicinal Chemistry 2008; 51(9): 2589-2599.
- Newman DJ, Cragg GM and Snader KM: Natural products as sources of new drugs over the period 1981–2002. Journal of Natural Products 2003; 66(7): 1022-1037.
- Suffness M and Douros J: Miscellaneous natural products with antitumor activity. In: Cassady JM, Douros JD. Anticancer Agents Based on Natural Product Models. New York, Academic Press 1980; 474 (Chapter 14).
- Sisodiya PS: Plant-derived anticancer agents: a review. International Journal of Research and Development in Pharmacy and Life Sciences 2013; 2(2): 295.
- Mycek JM and Harvey ARP: Lippincott's Illustrated Reviews: Pharmacology. Anticancer drugs Chapter 38, 392.
- Cragg GM and DJ Newmann: Journal of Ethnopharmacology 2005; 100: 72-79.
- Pinar R: To investigate the knowledge of Turkish people about cancer. Cancer agenda 1998; 2: 66-73
- Kainsa S, kumar P and Rani P: Medicinal plants of Asian origin having anticancer potential: a short Asian Journal of Biomedical and Pharmaceutical Science 2012; 2.
- Vukovic-Gacic B, Nikcevic S and Beric-Bjedov T: Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem Toxicol 2006; 44: 1730-1738.
- ÖnayUçar E, Karagöz A and Arda N: Antioxidant activity of Viscum album Fitoterapia 2006; 77: 556-560.
- Pinney KG, Jelinek C and Edvardsen K: The discovery and development of the combretastatins. In: Cragg GM, Kingston DGI, Newman DJ. Anticancer Agents from Natural Products. Taylor & Francis Group, Boca Raton, FL Brunner-Routledge Psychology Press 2005; Chapter 3, 23-46.
- Evandri MG, Battinelli L and Daniele C: The antimutagenic activity of Lavandula angustifolia (lavender) essential oil in the bacterial reverse mutation assay. Food Chem Toxicol 2005; 43: 1381-1387.
- Hwang BY, Su BN and Chai H: Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaiasilvestris. J Org Chem 2004; 69(10): 3350-3358.
- Cassady JM, Chan KK and Floss HG: Recent developments in the Maytansanoid antitumor agents. Chem Pharma Bulletin 2004; 52: 1-26.
- Suffness M and Douros J: Miscellaneous natural products with antitumor activity. In: Cassady JM, Douros JD. Anticancer Agents Based on Natural Product Models. New York , Academic Press 1980; Chapter 14,
- Ravelo AG, Estevez-Braun A and Chavez-Orellana H: Recent studies on natural products as anticancer agents. Curr Topics Med Chem 2004; 4: 241-265.
- Jang DS, Park EJ and Hawthorne ME: Potential cancer chemopreventive constituents of the seeds of Dipteryx odorata (tonka bean). J Nat Prod 2003; 66(5): 583-587.
- Denmeade SR, Jakobsen CM and Janssen S: Prostate-specific antigen-activated thapsigargin prodrug as a targeted therapy for prostate cancer. J Natl Cancer Inst. 2003; 95: 990-1000.
- Newman DJ, Cragg GM and Holbeck S: Natural products as leads to cell cycle pathway targets in cancer chemotherapy. Curr Cancer Drug Targets 2002; 2: 279-308.
- Hernandez-Ceruelos A, Madrigal-Bujaidar E and de la Cruz C: Inhibitory effect of chamomile essential oil on the sister chromatid exchanges induced by daunorubicin and methyl methanesulfonate in mouse bone marrow. Toxicol Lett 2002; 135: 103-110.
- Silva GL, Cui B and Chavez D: Modulation of the multidrug-resistance phenotype by new tropane alkaloid aromatic esters from Erythroxylum pervillei. J Nat Prod 2001; 64(12): 1514-1520.
- Lee D, Bhat KPL and Fong HHS: Aromatase inhibitors from Broussonetia papyrifera. J Nat Prod 2001; 64(10): 1286-1293.
- Marnewick JL, Gelderblom WCA and Joubert E: An investigation on the antimutagenic properties of South African herbal teas. Mutat Res 2000; 471: 157-166.
- Abdulla M and Gruber P: Role of diet modification in cancer prevention. Biofactors 2000; 12: 45-51.
- Dinkova-Kostova AT and Talalay P: Persuasive evidence that quinine reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free RadicBiol Med 2000; 29(3-4): 231–240.
- Sausville EA, Zaharevitz D and Gussio Z: Cyclin-dependent kinases: initial approaches to exploit a novel therapeutic target. Pharmacol Thera 1999; 82: 285.
- He K, Valcic S and Timmerman BN: Indole alkaloids from Aristoteliachilensis (Mol.) Stuntz. Int J Pharmacog 1997; 35: 215-217.
- Khamis SB, Brown JE and Bibby MC: Evaluation of two plants species used in Malay traditional medicine for the treatment of breast cancer. J Pharma Pharmacol 1997; 49: 113.
- Liang HR, Vuorela P and Vuorela H: Isolation and immunomodulatory effect of flavonol glycosides from Epimedium humaneness. Planta Medica 1997; 63: 316- 319.
- Peres MTLP, Monache FD and Cruz AB: Chemical composition and antimicrobial activity of Croton urucuranaBaillon (Euphorbiaceae). J Ethnopharmacol 1997; 56: 223-226.
- Barakat HH, Hussein SAM and Marzour MS: Polyphenolic metabolites of Epilobiumhersutum. Phytochemistry 1997; 46: 935-941.
- Bai L, Yamaki M and Takagi S: Lignans and a bichroman from Pleionebulbocodioides. Phytochemistry 1997; 44: 341-343.
- Jang M, Cai L and Udeani GO: Cancer chemopreventive activity of resveratrol, a natural product derived from grapes 1997; 275(5297): 218-220.
- Gupta MP, Monge A and Karikas GA: Screening of Panamanian medicinal plants for brine shrimp toxicity, crown gall tumor inhibition, cytotoxicity and DNA intercalation. Int J Pharmacol 1996; 34: 19-27.
- Suh HW, Song DK and Son KH: Antinociceptive effect of Smilaxin B administered intracerebroventricularly in the mouse. Planta Med 1996; 62: 141-145.
- Hoyoku N, Michiaki M and Harukuni T: Cancer prevention by carotenoids. Arch Biochem Biophys 2009; 483(2): 165-168.
- Wan X, Luo M and Li X: Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact 2009; 181(1): 15-19.
- Caihua L, Michael DMc and Carmen M: A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res 2009; 2(8): 759-768.
- Shiu-Chen H, Chi-Tang H and Shoei-Yn L: Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol 2005; 69(2): 221-232.
- Li M, Zhang Z and Hill DL: Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res 2005; 65(18): 8200-8.
- Ruiz D, Egea J and Tomas-Barberan FA: Carotenoids from new apricot (Prunus armeniaca) varieties and their relationship with flesh and skin color. J Agric Food Chem 2005; 53: 6368-74.
- Kim GY, Kim JH and Ahn SC: Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor. Kappa B. Immunolo 2004; 113(2): 203-11.
- Sunila ES and Kuttan G: Immunomodulatory and antitumor activity of Piper longum Linn. And piperine. J Ethnopharmacol 2004; 90 (2-3): 339-346.
- Shishodia S, Majumdar S and Banerjee S: Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res 2003; 63(15): 4375-83.
- Takada Y and Aggarwal BB: Betulinic acid suppresses carcinogen-induced NF-kappa B activation through inhibition of I kappa B alpha kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol 2003; 171(6): 3278-86.
- Cheng YH, Shen TF and Pang VF: Effects of aflatoxin and carotenoids on growth performance and immune response in mule ducklings. Comp Biochem Physiol Toxicol Pharmacol 1999; 128: 19-26.
- Abdullaev FI: Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus).Exp Biol Med 2002; 227: 20-5.
- Kohno H, Marda M and Honjo S: Prevention of colonic preneoplastic lesions by the beta-cryptoxanthin and hesperidin rich powder prepared from Citrus Unshiu Marc. Juice in male F344 rats. J Toxicol Pathol 1999; 12: 209-215.
- Han SS, Keum YS and Seo HJ: Capsaicin suppresses phorbol ester-induced activation of NF-kappaB/Rel and AP-1 transcription factors in mouse epidermis. Cancer Lett 2001; 164(2): 119-26.
- Manna SK, Mukhopadhyay A and Aggarwal BB: Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 2000; 164(12): 6509-19.
- Cheng YH, Shen TF and Pang VF: Effects of aflatoxin and carotenoids on growth performance and immune response in mule ducklings. Comp Biochem Physiol Toxicol Pharmacol 1999; 128: 19-26.
- Berkarda B, Koyuncu H and Soybir GT: Inhibitory effect of hesperidin on tumor initiation and promotion in mouse skin. Res Exp Med 1998; 198: 93-99.
- Laughton MJ, Evans PJ and Moroney MA: Inhibition of mammalian 5-lipoxygenase and cyclooxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and iron ion reducing ability. Biochem Pharmacol 1991; 42(9): 1673-81.
- Soliman KF and Mazzio EA: In-vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc Soc Exp Biol Med 1998; 218(4): 390-7.
- Kumar A, Dhawan S and Aggarwal BB: Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, Ikappa B degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene 1998; 17(7): 913-8.
- Sueoka N, Sueoka E and Okabe S: Anti-cancer effects of morphine through inhibition of tumor necrosis factor-a release and mRNA expression. Carcinogenesis 1996; 17(11): 2337-2341.
- Gogate SS: Cytotoxicity of neem leaf extract: an antitumor. Natl Med J India 1991; 9: 297.
How to cite this article:
Haque MU, Ferdiousi N and Sajon SR: Anti-cancer agents derived from plant and dietary sources: a review. Int J Pharmacognosy 2016; 3(2): 55-66. doi: 10.13040/IJPSR.0975-8232.3(2).55-66.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
55-66
740
2894
English
IJP
M. U Haque *, N. Ferdiousi and S. R. Sajon
Department of Pharmacy, Faculty of Biological Science and Technology, Jessore University of Science and Technology, Jessore, Bangladesh.
uzzalphr07@gmail.com
20 October 2015
23 January 2016
27 January 2016
10.13040/IJPSR.0975-8232.IJP.3(2).55-66
29 February 2016