ANTHELMINTIC ACTIVITY OF LEAVES EXTRACT OF ANDROGRAPHIS PANICULATA NEES
HTML Full TextANTHELMINTIC ACTIVITY OF LEAVES EXTRACT OF ANDROGRAPHIS PANICULATA NEES.
J. Murali 1, R. Maheswari 1, M. Syed Muzammil * 2 and G. Asogan 3
Department of Biochemistry 1, K.M.G. College of Arts & Science, Gudiyattam, Vellore - 635803, Tamil Nadu, India.
Department of Biochemistry 2, Islamiah College (Autonomous), Vaniyambadi - 635752, Tamil Nadu, India.
Department of Biochemistry 3, M.G.R. College of Arts & Science, Hosur - 635130, Tamil Nadu, India.
ABSTRACT: The present study is part of the continuing programme related to the antihelminthic activity and phytochemical screening of Andrographis paniculata Nees. (Kalmegh) and their enhanced potential on helminths. The antihelminthic activity of the aqueous extract of Andrographis paniculata Nees. leaves against the adult Indian earthworms Pheretima posthuma due to its anatomical and physiological resemblance with the intestinal roundworm parasites of human beings. Several plant species are administered orally to control the various diseases in our country. Some of these plants have been pharmacologically provided to be of some value and maybe a popular remedy for the treatment of various ailments. The growth of knowledge to cure disease continues at an accelerating pace and number of new plant-derived drugs increase likewise. Herbal medicine is currently experiencing a revival in Western society along with other complementary therapies such as traditional Chinese Medicines, Osteopathy and Homeopathy. In this context, the present study is the first milestone with particular emphasis on the application of Andrographis paniculata Nees. (Kalmegh) the medicinal plant, their antihelminthic effect, and phytochemical screening, for their better formulation and controlling the various diseases in future. The aqueous extract was found to be more potent, and activities are compared with the drug piperazine citrate as a reference drug.
| Keywords: |
Andrographis paniculata Nees., Piperazine citrate, Ethanol, Pheretima posthuma
INTRODUCTION: The use of environmentally benign materials like plant leaf extract, bacteria, and fungi as medicines represent the biggest human use of the natural world. Plants provide the predominant ingredients of medicines in most medical traditions. Plants have been used in traditional medicine for several thousand years.
The knowledge of medicinal plants has been accumulated during many centuries based on different medicinal systems such as Ayurveda, Unani, and Siddha. In India, it is reported that traditional healers used 2500 plant species and that 100 species of plants served as regular sources of medicine. In this scenario, herbal plants are gaining paramount importance in the synthesis of Herbal-medicines.
Given the above considerations, the present study was designed to investigate the protective effect of Andrographis paniculata Nees. (Kalmegh) their antihelminthic effect. Moreover, phytochemical screening was also done.
Anthelmintic: The substance which destroys or prevents the development of parasitic worms, such as filariae, flukes, hookworms, pinworms, round worms, schistosomes, tapeworms, trichinae, and whipworms. An anthelminthic drug may interfere with the parasites carbohydrate metabolism, inhibit their respiratory enzymes, block their neuro-muscular action, or render them susceptible to destruction by the host's macrophages. Drugs used in specific treating helminthic infections viz., piperazine, pyrantel pamoate, pyrvinium pamoate, mebendazole, niclosamide, hexylresorcinol, diethylcarbamazine, and thiabendazole. Before, the administration of a toxic anthelminthic it is customary to starve the patient for from twelve to twenty-four hours and to give a brisk cathartic, the object being to clean out the intestines and leave the worm in an exposed condition. The dose is then administered and is followed in four or five hours by a brisk, rapidly acting cathartic, such as castor oil or salts, to carry out the worm. Castor oil has been objected to on the ground that an oily medium will promote the absorption of the poison by the patient. This may be true, especially in the case of oleoresin of male fern, if rapid evacuation of the bowels does not take place.
Andrographis Paniculata Nees. (Kalmegh) belongs to the family of Acanthaceae and is popular worldwide with the name of “King of Bitters” in English. It is an annual herbaceous plant which is widely cultivated in Southern Asia, India, China and some parts of Europe. Some of the common names are the Kalmegh, Kalafath, Kan-jang, Alui, Charita, Cherota, Chiraita, Cheretta, Kariyat, Green Chiretta, Halviva, Kreat, Sinta, Rice bitters, Sambilata, Sambiloto, Kraut, Andrographidis. The leaves and roots have traditionally been used over the centuries in Asia and Europe as a folklore medicine for a wide variety of ailments or as herbal supplements for health promotion. Andrographis paniculata (Kalmegh) has an important place in the Indian Pharmacopoeia and is one of the most widely used plants in Ayurvedic formulations 1. The whole plant has a variety of therapeutic values. It has immunosuppressive properties and is useful in the treatment of wounds, ulcers, leprosy, sore throat, and hypertension 2. Panchang (stem, leaves, flowers, root, and seeds) of the plant is being used in the various formulation of Indian system of medicine for the treatment of fever, malaria and sore throat 3.
Andrographis paniculata has been used in the treatment of some skin infections in India by folkloric medicine practitioners. It is considered beneficial to the skin and is used both internally and externally for this purpose. The plant is also reported effective against diarrhea 4, and it is commonly used to prevent and treat common cold5. It has also been used traditionally for the sluggish liver as an antidote in case of colic dysentery and dyspepsia 6. The plant contains some diterpenoids. However, the major bitter constituent is andrographolide, which is diterpene lactone. Diterpenoids and flavonoids are the main chemical constituents of Andrographis paniculata which are believed to be responsible for the most biological activities of this plant 7. Two flavonoids, identified as 5,7,2’,3’-tetramethoxyflavonone and 5-hydroxy-7,2’,3’-trimethoxyflavone, as well as several other flavonoids were obtained from the whole plant. The bitter principle andrographolide was isolated in pure form by Goiter.
Andrographolide is also attributed with some other activities like liver protection anticancer activity anti-diabetic activity 8 and anti-malarial activity 8. The plant extracts exhibit anti typhoid, antifungal, antiviral 9 and anti-pyretic 10 activities. It is also reported to possess anti-inflammatory and anti-snake venom properties 11. Recent research has thrown light on the cultivation of this plant on a large scale because of its high medicinal value. Hence, the present investigation was taken up with an objective to evaluate the antimicrobial potential against the microorganisms.
MATERIALS AND METHODS:
Chemicals: All the fine chemicals were purchased from Sigma chemical co., USA. All other chemicals used were of good quality and analytical grade.
Phytochemical Analysis:
Qualitative Chemical Examination (Aqueous Extract):
Alkaloid: Meyer’s test (potassium mercuric iodide-1.36g of mercuric chloride was dissolved in 60 ml of distilled water and 5 g of KI in 10ml of water. The 2 solution were mixed and diluted to 100 ml with distilled water). To 1 ml of acidic aqueous solution of samples, few drops of reagent were added. Formation of white or pale precipitate showed the presence of alkaloid.
Test for Proteins: To the test solution the Biuret reagent is added. The blue reagent turns violet in the presence of proteins.
Test for Sugars: The substance was mixed with an equal volume of Fehling’s A and B solutions, heated in a water bath. Formation of red color is the indication of the presence of sugar.
Tannins: In a test containing 5ml of extracts a few drops of 1% solution of lead acetate was added. A yellow or red precipitate was formed including the presence of tannins (Lead acetate test).
Test for Coumarin: To the test sample 10% of sodium hydroxide and chloroform were added. Formation of yellow color indicates the presence of coumarin.
Test for Gum: To the substance, add a few ml of water and shake well. Formation of swells or adhesives indicates the presence of gum.
Saponins: In a test tube containing about 5 ml of an extracts a drop of sodium bicarbonate was added. The mixture was shaken vigorously and kept for 3 min. A honeycomb-like froth was formed, and it showed the presence of saponins.
Resin: To 2 ml of extract 5-10 ml of acetate anhydride was added, dissolved by gently heating code and then 0.5 ml of sulphuric acid was added. The bright purple color was produced. It indicates the presence of resins.
Flavonoids: In a test tube containing 0.5ml of an extract of the sample 5-10 drops of dilute HCl and a small piece of Zn or Mg were added, and the solution was boiled for few minutes. In the presence of flavonoids, reddish-pink or dirty brown color was produced.
Glycosides: A small amount of extract was dissolved in 1 ml of water, and then aqueous sodium hydroxide solution was added. Formation of yellow color indicates the presence of glycosides.
Steroids: To 2 ml of an extract of sample 1ml of concentrated sulphuric acid was added carefully along the sides of the test tube. A red color was produced in the chloroform layer.
Phenols: To 1 ml of extract 2 ml of distilled water followed by a few drops of 10% aqueous ferric chloride solution were added. Formation of blue or green indicates the presence of phenols (Ferric chloride test).
Terpenoid: 2 ml of concentrated sulphuric acid was added to 1 mg of extract and observed for the reddish brown color.
Cardiac Glycoside: Total 100 mg of the extract was dissolved in 1 ml of glacial acetic acid containing one drop of ferric chloride solution. This was then under layered with 1 ml of concentrated sulphuric acid a brown ring obtained at the interface indicates the presence of deoxysugar characteristics of cardenolides.
Test for Quinones: To the test substance, sodium hydroxide was added. Blue-green or red color indicates the presence of quinone.
Triterpenoids: 10 mg of the extract acetic acid was added following the addition of concentrated sulphuric acid. Formation of reddish-violet color indicates the presence of triterpenoids.
The Plant Materials: Fresh leaves of the Andrographis Paniculata Nees plant were collected from Vellore, Tamil Nadu, India. After authentication, fresh leaves of the plant was collected in bulk, washed under running tap water to remove the dust adhered, then shade dried and pulverized in a mechanical grinder. The powder was passed through sieve no. 40 and used for extraction. The Andrographis paniculata Nees leaves extract was abbreviated as APALE.
Preparation of Plant Extract: The powdered leaves were extracted separately with distilled water (containing chloroform 0.25% v/v as a preservative) and hydro-alcohol (water: alcohol, 1:1) by continuous hot percolation for 72 h. The solvents were removed by distillation. The dried extracts (residue) were suspended in normal saline and used for the anthelminthic activity. The percentage of yield was found to be 10%.
Preparation of Test Samples: Test samples were prepared by dissolving and suspending 2.5 g of extract in 25 ml of distilled water to obtain a stock solution of 100 mg/ml. From this stock solution, different working dilutions were prepared to get a concentration range of 25 and 50 mg/ml final volume made with saline solution.
Experimental:
Parasites Recovery: Anthelmintic activity was carried as per the method reported by 12 with minor modifications. Indian Adult earthworms (P. posthuma) were collected from moist soil. Earthworms are 5-7 cm in length and 0.1-0.3 cm in width, were washed with normal saline to remove all the fecal matter and collected in 0.9% neutral phosphate buffered saline (PBS, pH 7) and then incubated at 37 ± 1 °C in a glass-chambered digital BOD incubator. 1 h before in vitro experimental assay, different concentrations of the extract, viz., 10, 25, and 50 mg/ ml, were prepared by dissolving them in 0.9% PBS, supplemented with 1% dimethylsulfoxide (DMSO), and that were all maintained at 37 ± 1 °C. The fresh worms were directly introduced to the different concentrations of the plant extract in separate petri dishes. Similar treatment was performed for piperazine citrate as a reference drug at a concentration of 15 mg/ml.
The petri dishes were introduced with worms. One group is maintained in a medium containing only PBS with 1% DMSO as a control. Each experiment consisted of 6 replicates. Each petridish was placed with 6 worms and observed for paralysis (or) death. Observations were made for the time taken to paralyze and death of individual worms. Paralysis was said to occur when the worms do not move even in normal saline. Death was concluded when the worms lost their motility followed with fading away of their body color 4.
Motility & Mortality Studies: Motility of the worms were observed, the time taken for paralysis and death was recorded, as previously described 7. Paralysis is defined as complete loss of movement upon physical provocation of the worms. Dipping the parasites in tepid PBS (~45oC) induced movement in sentient worms; if no movement occurred upon such stimulation, death was confirmed. Observations were made for the time taken to paralyze and death of individual worms. The time for paralysis was noted when no movements could be observed except when the earthworms are shaken vigorously. Mortality was concluded when the earthworms lost their motility followed with the fading away of their body colors. We carried out the experiments for Ascardia galli worms too, with the different solution prepared in normal saline.
RESULTS AND DISCUSSION: The efficacy of plant extract and the drug on the viability of the parasite are represented in Table 1. Pheretima posthuma maintained as the control in only PBS with 1% DMSO survived very well up to 159.83 ± 0.70 h. The results indicate that the plant extract showed dose-dependent lethal effect.
TABLE 1: PHYTONUTRIENTS SCREENING OF THE AQUEOUS EXTRACT OF A. PANICULATA NEES.
| Tests | Extract of Andrographis paniculata Nees. |
| For Carbohydrates | |
| Molisch’s Test
Benedict’s Test |
+
+ |
| For Amino Acids | |
| Ninhydrin Test | - |
| For Alkaloids | |
| Dragendroff’s Test
Mayer’s Test |
-
- |
| For Sterols
Libermann reaction |
+ |
| For Triterpenoids | |
| Salkowski Test | - |
| For Phenolics & Tannins | |
| Lead acetate Test | + |
| For Flavonoids Glycosides | |
| Shinoda Test
Alkaline Test |
+
+ |
| For Saponins Test | |
| Foam Test | - |
‘+’ – Positive, ‘-' – Negative
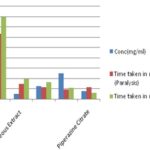
TABLE 2: ANTIHELMINTHIC EFFECT OF ANDROGRAPHIS PANICULATA NEES AQUEOUS LEAF EXTRACT
| S.
no. |
Test Group Conc./Dose(mg/ml) | Time taken in Min | ||
| Paralysis | Death | |||
| 1 | Control | 0 | 126.46±0.70 | 159.83±0.70 |
| Aqueous Extract | 10 | 29.76±0.38 | 39.22±0.57 | |
| 25 | 22.48±0.50 | 32.61±0.60 | ||
| 50 | 17.92±0.59 | 21.17±0.74 | ||
| 2 | Ethanolic Extract | 10 | 25.39±0.44 | 34.97±0.47 |
| 25 | 20.50±0.53 | 26.43±0.59 | ||
| 50 | 16.87±0.48 | 19.71±0.64 | ||
| 3 | Piperazine Citrate | 15 | 23.29±0.61 | 12.29±0.54 |
Results are expressed as Mean ± SEM.
GRAPH 1: ANTIHELMINTHIC EFFECT OF A. PANICULATA NEES. AQUEOUS LEAF EXTRACT
Those incubated with 10, 25, and 50 mg of Andrographis paniculata Nees. leaf aqueous extract per ml of PBS showed complete loss of life in 39.22 ± 0.57, 32.61 ± 0.60 and 21.17 ± 0.74 min. Piperazine citrate took 23.29 ± 0.54 min for 15 mg/ml, to kill the worms effectively. Though the time is taken for each concentration of the plant extract to effectively kill the parasite comparatively longer than that for the reference drug, the results are highly significant. It becomes apparent that the anthelmintic activity is inversely proportional to the time taken for paralysis/death of the worms. Our work would have paved the way for further interest to isolate the possible constituents those responsible for anthelmintic activity and study their potency against other helminths shortly.
CONCLUSION: Our study summaries plant derived incorporated antibiotics which are widely prescribed world-wise and are considered natural, safe, and beneficial. Interest has revived recently in the investigation of medicinal plants to identify novel active phytochemicals that might lead to drug development as more potent antihelminthics, antibiotics, antimalarials, anticancer agents, etc derived from research on plants. Antihelminthic effect of the extract may be attributed to the phytochemical constituents such as sterols and terpenes, polyphenols, flavonoids, tannins saponins, and alkaloids. These compounds would be having strong activity due to the phytochemicals and could explain the antihelminthic activity of the plant. However, the more detailed phytochemical analysis is required to isolate and characterize each active compound which is responsible for the antihelminthic activity and exact mechanisms of action of this activity. The allopathic forms of antihelminthics give adverse effects to the human system and the metabolism. So, therefore we need antihelminthics which have lower side effects and high effectiveness. The combination of the high amount of herbal extracts and low amount of drugs will give new path in the medicinal world. It is a new concept to combine herbal and Allopathy drugs to be known as herbo allopathy combinations shortly.
ACKNOWLEDGEMENT: We are grateful to the faculty members of both the colleges, PG and Research Department of Biochemistry & Micro-biology, Principal, Islamiah College and K.M.G College, Principal, Secretary and Correspondent, Islamiah College, General Secretary and VMES for their encouragement and support in carrying out the work.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Aleemuddin MA, Karthikeyan M and Rajasekar S: Int J Pharma Sci Rev Res 2011; 11: 133-136.
- Ali M, Bhutani KK and Srivastava TN: Phytochemistry 1990; 29(11): 3601.
- Ambasta SP: The useful plants of India. CSIR Publication, New Delhi, India, 1986: 705.
- Arnold MD and Harry L: Poisonous Plants of Hawaii. Tokyo, Japan: Charles E. Tuttle Co. 1968: 51.
- Arul B, Kothai R, Kumar KS and Christiana AJM: Pak J Pharm Sci 2005; 18(3): 17-20.
- Yadav AK, Tandon V and Rao HSP: Fitoterapia 1992; 13(5): 395-398.
- Beena P, Purnima S and Kokilavani R: Der Pharmacia Lettre 2011; 3(4): 320-324.
- Biswal PR, Sardar KK, Parija SC, Mishra PR and Mishra SN: Indian Journal of Indigenous Medicines 1977; 19(1): 71-74.
- Carvalho LH, Brandao MG, Santos-Filho D, Lopes JL and Kretti AV: Brazilian Journal of Medical and Biological Research 1999; 24(11): 1113-1123.
- Cauis JF: The Medicinal and Poisonous Plants of India. Scientific Publishers, Jodhpur, India, 1998: 493.
- Chopra RN, Nayar SL and Chopra IC: Glossary of Indian Medicinal Plants, New Delhi, Council of Scientific and Industrial Research 1958: 74.
- Rajesh R, Chitra K and Paarakh PM: Int J Phar Sci & Drug Res 2010; 2(4): 269-271.
How to cite this article:
Murali J, Maheswari R, Muzammil MS and Asogan G: Anthelmintic activity of leaves extract of Andrographis paniculata Nees. Int J Pharmacognosy 2014; 1(6): 404-08. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(6).404-08.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
9
404-408
517
2624
English
IJP
J. Murali, R. Maheswari, M. S. Muzammil * and G. Asogan
Department of Biochemistry 2, Islamiah College (Autonomous), Vaniyambadi, Tamil Nadu, India.
syed_bio2004@yahoo.co.in
28 April 2014
21 May 2014
28 May 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(6).404-08
01 June 2014