AN OVERVIEW OF CUCUMIS MELO L.’S PHYSICOCHEMICAL TRAITS AND HEALTH-PROMOTING QUALITIES
HTML Full TextAN OVERVIEW OF CUCUMIS MELO L.'S PHYSICOCHEMICAL TRAITS AND HEALTH-PROMOTING QUALITIES
Kiran P. Gaikwad *, Mahesh B. Narkhede, Jaya P. Ambhore and Chanchal S. Chandak
Department of Pharmacy, Dr. Rajendra Gode College of Pharmacy, Malkapur, Maharashtra, India.
ABSTRACT: Despite its succulent fruit, Cucumis melo L. is a widely grown crop in many parts of the world. Developing consumer-favored cultivars requires an understanding of the genetic foundation of a plant's qualitative and quantitative features. Cucumis melo L. is a delightful oval-shaped fruit that belongs to a particular cultivar group of muskmelons. It is the most exported and consumed fresh fruit and is consumed widely in many tropical nations. It has incredible nutritional value. It was evaluated how different organic solvents affected the phytochemical recovery from the fruits and seeds of the Cucumis melo L. plant. Its widespread cultivation is attributable to its adaptability to many soil types and climates. Because of its complex chemical makeup and pleasant taste, melons are a great source of biologically beneficial chemicals for humans. Melon contains glucose, fructose, vitamin A, thiamine, D, C, K, and E, as well as a few vitamins from group B. Aqueous or fresh extracts of the edible portion of melon fruit have long been employed as analgesics, purgatives, analgesics for painful discharges, analgesics for dysuria, and anti-inflammatory agents. As of right now, a small number of studies on melon pulp, peels, and seeds have demonstrated the fruit's several beneficial biological qualities, including provitamin A, antioxidant, anticancer, and antibacterial activity. In this paper, we provide a summary of the nutritional characteristics and bioactive substances found in melon fruit.
Keywords: Cucumis melo L., Bioactive substances, Fruit
INTRODUCTION: Melon (Cucumis melo L) (2n = 2x = 20 four), a member of the Cucurbitaceae family, is a vital eudicot diploid with a genome size of 454 Mb 1. It is widely grown in temperate, subtropical, and tropical regions of the world. The countries that produce the most melon are China, the United States, Spain, Turkey, and Iran 2.
Melon exhibits significant variety in its physical, biochemical, and behavioral properties, depending on the climatic zones and local preferences 3. Australia, Africa, and Asia are home to melon's wild cousins 4-7.
Fresh melon fruits exhibit significant variations in their morphology, including variations in shape, color, texture, and flavor 8. Melon fruit can range in size from spherical to long form, with a maximum weight of 20 kg. Fruits can range in flavor from bland to sweet, acidic, or even bitter 9, 10. A number of hybrids have been created recently through breeding with the goal of improving disease resistance, abiotic stress resistance, and storage and shelf life. Cultivars with longer shelf lives (LSL kinds) are generally are viewed as having poor fruit quality by consumers and, as a result, have a minimal acceptability 11. Nonetheless, the majority of breeders agree that farmers' basic needs for modern melon cultivars include fusarium and powdery mildew resistance 12. Additionally, a key factor that improves commercial productivity is insect pest management, which offers improved yield safety measures and decreased waste in addition to far superior fruit quality 13.
In Europe and Asia, the melon leaf curl New Delhi virus is causing a large crop loss. More recently, a QTL for melon disease resistance was discovered 14. Comparably, melon's quantitative genes for yield and yield-related features have been extensively researched, in contrast to other cucurbits as bitter gourd 15. Moreover, numerous researchers have revealed that even in the face of biotic and abiotic stress, melon's capacity for merging and the benefits of heterosis in fruit output, along with other morphological traits, are still present 16, 17. Melon can now be sequenced using next-generation technology because of its excellent sensitivity and incredibly low cost. Particularly, multiplexed sample RAD sequencing and genotyping by sequencing (GBS) are accessible, quick, and reproducible 18-20.
Variety: Cucumis melo belongs to family Cucurbitaceae is a horticultural crop of great economic importance and showed a wide range of diversity for agro-morphological and fruit traits 3. Leaves are simple, three or five-lobed, borne singly at nodes, have significant variation for colour, shape and size. Tendrils are simple and borne on leaf axils. Variations in melon fruits were observed for shape, size, internal and external colour. Melon fruits are typically of the climacteric type, but the inodorus variety was found to have non-climacteric fruit 8. Flesh color ranged from orange, light orange, pink, white, and green; rind color ranged from green, white, orange, yellow, and red grey; rind texture as smooth, striped, warty, rough, and netted; shape varied from round, elongated, flattened; size 4 cm in C. melo var. agrestis to 200 cm in C. melo var. flexuosus 9, 21, 22. The length to breadth ratio of almost all melon fruits is 1:1, however measurements show that flexuosus kinds have a width to length ratio of about 4:1 23.
Due to the appearance of so many wild Cucumis species there has been suggestion that Africa is the origin of cultivated melon. However, recent research 6, 7, 24 indicate that the closest wild relatives of melon are located in Australia and India. It consists of fifteen distinct groups or variants, ten of which are associated with the species species melo. These include cantalupensis, reticulatus, adana, chandalak, ameri, and inodorus.
The species S. agrestis includes chate, flexuosus, dudaim, and tibish, as well as five variants that include momordica, conomon, chinensis, makuwa, and acidulous 25. One of the most diverse genera in the family Cucurbitaceae is the genus Cucumis. Pitrat has given Melo ssp melo assignments to 11 groups. Furthermore, melon is renowned for exhibiting variation in terms of their postharvest ripening characteristics. Variations in the rates of ethylene production could be the cause of this. Researchers' ideas have led to a dynamic development in the classification of melons 26. In Iran, India, and China, melons have a lengthy history of development. Melon trading was a common practice along the Silk Road, connecting East and West 27.
During it is suggested that the center of melon variety lies in both Asia and Africa. The melon had a division into two lineages approximately 2 million years ago. Contemporary market kinds in melon are a product of the Asian lineage, with one subspecies (C. melo subsp. Melo) restricted to Asia and the other (C. Melo subsp. meloides) restricted to Africa 7. In contrast, three domestication episodes involving melon have been documented, two of which occurred in India and the other one in Africa, according to the most comprehensive study utilizing 1175 accessions resequencing data 28. Yet, phylogenetic categorization based on metabolic data gained popularity in the last century. Studying melon species at the interspecific level, which is typically based on single biochemical families like alkaloids, was the main focus at that period. In light of the development this tendency has shifted to using genotyping techniques along with metabolomics, such as NMR, GC-MS, LC-MS, and so on. In order to classify the genotypes more precisely, 29 recently carried out the metabolomics analysis.
Cucumis melo L. Peel Bioactive Ingredients: Peel extract from Cucumis melo L. (maazoun cultivar) was shown to contain 18 distinct phenolic compounds, according to Mallek-Ayadi et al. (2016) 30. The means of high-performance liquid chromatography (HPC). Nine kinds of phenolic compounds hydroxybenzoic acids, phenyl-ethanoids, phenolic alcohol, hydroxycinnamic acids, flavones, flavanone glycosides, secoiridoids, benzeneacetic acid, and lignin were identified among them. With 33.45±0.37 mg/100g, 3-hydroxybenzoic acid was the predominant phenolic compound. It was followed by luteolin-7-glycoside (16.51±0.15 mg/100g), m-coumaric acid (19.91±0.37 mg/100g), apigenin-7-glycoside (29.34±0.17 mg/100g), and isovanillic acid (23.70±0.04 mg/100g). Besides, notable amounts of flavone (13.51±0.32 mg/100g), gallic acid (12.07±0.12 mg/100g), naringenin (11.58±0.11 mg/100g), and tyrosol (11.35±0.03 mg/100g) were also present in the melon peel extract. Next, HPLC analysis on Cucumis melo L. var. cantalupensis peel extract revealed the four phenolic compounds of 4-hydroxybenzoic acid (326.2 µg/g dry weight), vanillin (197.4 µg/g dry weight), coumaric acid (81.1 µg/g dry weight), and chlorogenic acid (65.9 µg/g dry weight) 31. Another study investigated the phenolic compounds present in sharlyn melon peel powders, whereby four phenolic compounds were detected: 4-hydroxybenzoic acid (958.3 µg/g dry weight), vanillin (851.8 µg/g dry weight), coumaric acid (8.8 lg/g dry weight), and chlorogenic acid (66.2 µg/g dry weight) 32.
Cucumis melo L. Seed Contains Bioactive Substances: The seed extract of Cucumis melo L. (maazoun cultivar) contained 15 different phenolic components, including flavonoids, phenolic acids, and among the phenolic classes found were stilbenoid, secoiridoid, and phenolic monoterpene. Naringenin-7-O-glycoside (4.30±0.00 mg/100g) had the highest concentration of phenolic chemicals, followed by gallic acid (4.24±0.03 mg/100g), vanillic acid (3.87±0.02 mg/100g), and 4-hydroxybenzoic acid (3.28±0.03 mg/100g) [33]. Concurrently, the chemical evaluation of the tocopherol content in three different melon types (honeydew, dessert 5, etc.) and hybrid 1) seed oils showed that γ-tocopherol, β-tocopherol, γ-tocotrienol, and γ-tocopherol were present. γ-tocopherol had the highest content among these three melon varieties, ranging from 71.4±0.3% to 91.5±0.5% 34. Additionally, Cucumis melo L. var. tibish seed oil has the greatest concentration of δ-tocopherol (27.40±0.53), according to Azhari et al. (2014) 35. mg/100g oil), γ-tocopherol (13.10±0.41 mg/100g oil), and α-tocopherol (2.70±0.17 mg/100g oil) in order of precedence. β-tocopherol was not found in this investigation, nevertheless. The phenolic components of Cucumis melo L. (maazoun cultivar) seed oil were then investigated by Mallek-Ayadi et al. (2017) 36, who detected 11 phenolic compounds. Amentoflavone had the highest level, measuring 32.80±0.21 µg/g fresh weight. It was followed by luteolin7-O glycoside, which had a value of 9.60±0.01 µg/g fresh weight. The results were: 4.72±0.01 µg/g fresh weight for naringenin and 7.26±0.02 µg/g fresh weight for gallic acid. β+γtocopherols (18.13±0.41 mg/100 g) accounted for the majority of the tocopherol content in the seed oils, with δtocopherol (6.09±0.53 mg/100 g) and α-tocopherol (2.85±0.17 mg/100 g) following closely behind.
Cucumis melo L. Flesh Contains Bioactive Substances: The study conducted by Laur and Tian (2011) 37 investigated the levels of β-carotene and vitamin C in cantaloupe and honeydew melons. That was noted that the melons with cantaloupe had more vitamin C and βcarotene than those with honeydew. We then investigated the β-carotene concentration of three rock melon cultivars: Champion, Glamour, and Honeymoon. According to Norrizah et al. (2012) 38, the product with the highest content was Honeymoon, with 9.5×10-4%, followed by Glamour (5.2×10–5%) and Champion (3.4×10–4%).
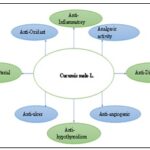
FIG. 1: THE MEDICINAL PROPERTIES OF CUCUMIS MELO L.
Important Pharmacological Properties:
Antioxidant Activity: Cucumis melo L.'s antioxidant activity was investigated in five investigations, with different methods such as 2,2-diphenyl – 1 - picrylhydrazylradical radical scavenging activity (DPPH RSA), hydroxyl radical scavenging activity (HRSA), hydrogen peroxide RSA, ferric reducing antioxidant power (FRAP), and 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity (ABTS RSA). Initially, 39 found that the extract of Cucumis melo L. var. cantalupensis had a DPPH RSA of 91.73±0.35%, 66.36±0.95%, and 48.55±0.84% for the skin, flesh and seed, respectively. Subsequently, the three rock melon cultivar seed and skin variants (Glamour, Champion, and Honeymoon) were subjected to the DPPH assay showed a SC50 ranging from 250 to 500 µg/mL, with Glamour seed exhibiting the highest RSA at the lowest dose 31. Additionally, a different study assessed the DPPH RSA and HRSA of a methanolic extract of Cucumis melo L.'s meat, seed, and skin. The HRSA varied from 37.37±2.42 dimethyl sulfoxide equivalents (DMSOE)/g extract to 67.19±8.90 g DMSOE/g extract, whereas the IC50 of DPPH RSA ranged from 9.58±0.37 mg/mL to 25.44±2.83 mg/mL 39.
Additionally, according to Mehra et al. (2015) 40, the FRAP of Cucumis melo L. seeds was 5.63 µg butylated hydroxytoluene (BHTE)/mg sample. With IC50 values of 23.30 mg/mL and 25.25 mg/mL in comparison to BHT (10.52 mg/mL and 14.05 mg/mL), respectively, the antioxidant activity of Seinat (Cucumis melo L. var. tibish) seed oil was also investigated utilizing the ABTS and DPPH assays 41. Furthermore, three investigations looked at the Cucumis melo L. var agrestis seed extract; in the investigations by Gill et al. (2011) and Sood et al. (2011) 42, the Cucumis melo L. var agrestis seed extract was exposed to DPPH and its RSA. The extract concentrations varied between 100 µg/mL and 300 µg/mL, with a range of 52.8±0.28% to 74.9±0.76%. Furthermore, RSA was shown to range from 24.01±7.1% to 75.59±6.7% when the same seed extract was used in another DPPH assay. The extract concentrations ranged from 50 µg/mL to 300 µg/Ml 43 reported that extract at 200 µg/mL to 400 µg/mL demonstrated hydrogen peroxide RSA levels ranging from 45.23±5.4% to 69.86±4.0%.
In summary, the root shake alcohol (RSA) of seed extract at concentrations ranging from 25 µg/mL to 200 µg/mL was found to be between 35.2±0.02% and 58.9±0.01% 42.
Anti-inflammatory and Analgesic Activity: In their 2011 study, Gill et al. 42 used carrageenan-induced paw edema in rats to examine the anti-inflammatory properties of Cucumis melo L. var agrestis seed extract. It is the mice's tail flick and immersion techniques were also used to look at analgesic activity 200 mg/kg and 300 mg/kg of seed extract, respectively, significantly reduced the amount of paw edema by 43.4% and 56.6%, with the larger dose producing a significant reduction in pain. Subsequently, utilizing the same methodology as the earlier work, 43 assessed the analgesic effect of the seed extract in albino rats by tail immersion and acetic-acid-induced jerking reaction in mice. Similar to the earlier study, the anti-inflammatory efficacy was also examined using showed findings indicating that the administration of 300 mg/kg of seed extract decreased the development of rat paw edema by 61.6%. In addition, the tail immersion method showed a considerable increase in pain threshold after 60 minutes at a dose of 300 mg/kg, whereas the acetic acid-induced writhing method demonstrated an analgesic effect of 70.6%.
Anti-bacterial Activity and Anti-ulcer Activity: The antibacterial properties of the essential oil isolated from Seinat (Cucumis melo L. var. tibish) were investigated in an in-vitro study carried out in China. Salmonella typhimurium, Shigella dysenterae, and Escherichia coli are three strains of Gram-negative bacteria and three strains of Gram-positive bacteria, namely Streptococcus pyogenes, Staphylococcus aureus, and Bacillus subtilis. The study's findings subsequently led to the conclusion that the extracted essential oil has antibacterial action against all types of bacteria, particularly Grampositive bacteria, with a minimum inhibitory concentration ranging from 0.5 to 5 mg/mL of sample 44. Likewise, pyloric ligation, water immersion stress, and nonsteroidal anti-inflammatory drug (NSAID)–induced ulcer models were used to evaluate the antiulcer effectiveness of Cucumis melo L. seed methanolic extract against stomach ulcerations. Indomethacin was one of the NSAIDs used in this experiment. The results showed that the seed extract prevented ulcers in water immersion stress, pyloric ligation and, after delivery at a dose of 300 mg/kg, NSAID-induced ulcer models by57.6%,67.6%, and 61.9%, respectively 45.
Anti-hypothyroidism, Anti-angiogenic and Anti diabetic Activity: A study carried out in-vivo on Wistar albino male rats that were both healthy and hypothyroid due to propylthiouracil showed notable increases in thyroid hormone (T3 and T4) levels after 100 mg/kg of peel extracts of Cucumis melo L. was administered. According to Parmar and Kar (2009) 46, this suggested that the peel extracts have thyroid-stimulating qualities.
In the meantime, a three-dimensional culture of human umbilical vein endothelial cells was used in an in-vitro investigation to investigate the antiangiogenic activity of a trypsin inhibitor isolated from Cucumis melo L. seeds.
The results showed that angiogenesis can be suppressed by a trypsin inhibitor 47, 48 looked at the function of Cucumis melo L. var. makuwa Makino, also known as the oriental melon, seeds on α-glucoside and α-amylase suppression. Hexane extract was found to inhibit α-glucoside and α-amylase by 35.5% and 61.8%, respectively, according to the results.
TABLE 1: THE BIOACTIVE COMPONENTS OF CUCUMIS MELO L.
| Country | Fruit part | Bioactive compounds | Ref. |
| Tunisia | Cucumis melo L. (maazoun cultivar) seeds | Naringenin-7-O-glycoside Gallic acid Vanillic acid 4-hydroxybenzoic acid | 30 |
| Tunisia | Cucumis melo L. maazoun (cultivar) seed oil | Amentoflavone Gallic acid Protocatechuic acid Caffeic acid Rosmarinic acids Luteolin-7-O glycoside α-tocopherol β+γ-tocopherols δ-tocopherol | 50 |
| Tunisia | Cucumis melo L. maazoun (cultivar) peels | 3-Hydroxybenzoic acid Apigenin-7-glycoside Isovanillic acid m-coumaric acid Oleuropein Luteolin-7-glycoside Gallic acid Tyrosol Naringenin Flavone | 36 |
| China | Cucumis melo L. var. tibish seed
C. melo L. var. tibish seeds |
δ- tocopherol γ-tocopherol α-tocopherol Hexenal | 35 |
| South Korea | C. melo L. var. makuwa Makino seed | Unsaturated fatty acid: palmitic acid, oleic acid and linoleic acid | 48 |
| India | Cucumis melo L. var. agrestis seeds | Triterpenoids, sterols | 42 |
| Egypt | Sharlyn melon Cucumis melo ( L.) peels | 4-hydroxybenzoic acid vanillin coumaric acid chlorogenic acid | 32 |
CONCLUSION: Cucumis melo L. showed a number of health benefits, especially when its by-products (seeds and peels) were consumed. Consequently, these byproducts may be added to a variety of food and nutraceutical applications to produce cutting-edge functional foods or dietary supplements.
To ensure efficacy and sustainability, research should be done on the sensory characteristics of the novel food products as well as the bioavailability of the bioactive chemicals throughout product development. By raising their quality of life, this can improve people's health and wellbeing. At the same time, Cucumis melo L. by-products might be used to address food waste, which became a significant problem.
ACKNOWLEDGEMENT: We are thankful to the Department of Pharmacology and Chemistry, Dr. Rajendra Gode College of Pharmacy, Malkapur for providing guidance and facilities.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Garcia-Mas J, Benjak A, Sanseverino W, Bourgeois M, Mir G, González VM, Hénaff E, Câmara F, Cozzuto L, Lowy E and Alioto T: The genome of melon (Cucumis melo). Proceedings of the National Academy of Sciences 2012; 109(29): 11872-7.
- Faostat F: Statistics division of food and agriculture organization of the United Nations 2018.
- Silberstein L, Kovalski I, Brotman Y, Perin C, Dogimont C, Pitrat M, Klingler J, Thompson G, Portnoy V, Katzir N and Perl-Treves R: Linkage map of Cucumis melo including phenotypic traits and sequence-characterized genes. Genome 2003; 46(5): 761-73.
- Luan F, Delannay I and Staub JE: Chinese melon (Cucumis melo) diversity analyses provide strategies for germplasm curation, genetic improvement, and evidentiary support of domestication patterns. Euphytica 2008; 164: 445-61.
- Kerje T and Grum M: The origin of melon, Cucumis melo: a review of the literature. In VII Eucarpia Meeting on Cucurbit Genetics and Breeding 2000; 510: 37-44.
- Sebastian P, Schaefer H, Telford IR and Renner SS: Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proceedings of the National Academy of Scienc 2010; 107(32): 14269-73.
- Endl J, Achigan‐Dako EG, Pandey AK, Monforte AJ, Pico B and Schaefer H: Repeated domestication of melon (Cucumis melo) in Africa and Asia and a new close relative from India. American Journal of Botany 2018; 105(10): 1662-71.
- Kirkbride JH: Biosystematic monograph of the genus Cucumis (Cucurbitaceae): botanical identification of cucumbers and melons. Parkway Publishers, Inc 1993.
- Liu L, Kakihara F and Kato M: Characterization of six varieties of Cucumis melo based on morphological and physiological characters, including shelf-life of fruit. Euphytica 2004; 135: 305-13.
- Monforte AJ, Oliver M, Gonzalo MJ, Alvarez JM, Dolcet-Sanjuan R and Arus P: Identification of quantitative trait loci involved in fruit quality traits in melon (Cucumis melo). Theoretical and Applied Genetics 2004; 108: 750-8.
- Wolff DW and Dunlap JR: Ethylene production rate and postharvest shelf-life diversity in melon (Cucumis melo) Germplasm. Hort Science 1995; 30(4): 827.
- Branham SE, Levi A, Katawczik M, Fei Z and Wechter WP: Construction of a genome-anchored, high-density genetic map for melon (Cucumis melo) and identification of Fusarium oxysporum f. sp. melonis race 1 resistance QTL. Theoretical and Applied Genetics 2018; 131: 829-37.
- Ramamurthy RK and Waters BM: Identification of fruit quality and morphology QTLs in melon (Cucumis melo) using a population derived from flexuosus and cantalupensis botanical groups. Euphytica 2015; 204: 163-77.
- Sáez C, Esteras C, Martínez C, Ferriol M, Dhillon NP, López C and Picó B: Resistance to tomato leaf curl New Delhi virus in melon is controlled by a major QTL located in chromosome 11. Plant Cell Reports 2017; 36: 1571-84.
- Kesh H, Kaushik P. Visiting Bitter Gourd (Momordica charantia) from a Breeding Perspective: A Review.
- Abou Kamer ME, Yousry MM and El-Gamal AM: Heterosis and heritability studies for fruit characters and yield in melon (Cucumis melo). Middle East Journal of Applied Sciences 2015; 5(1): 262-73.
- Singh V and Vashisht VK: Heterosis and combining ability for yield in muskmelon (Cucumis melo). Int J Curr Microbiol App Sci 2018; 7(8): 2996-3006.
- Ganal MW, Wieseke R, Luerssen H, Durstewitz G, Graner EM, Plieske J and Polley A: High-throughput SNP profiling of genetic resources in crop plants using genotyping arrays. Genomics of Plant Genetic Resources: Volume 1. Managing, Sequencing and Mining Genetic Resources 2014; 113-30.
- Shang J, Kong S, Li N, Wang J, Zhou D, Li N and Ma S: Genetic mapping and localization of major QTL for bitterness in melon (Cucumis melo). Scientia Horticulturae 2020; 266: 109286.
- Zhang H, Yi H, Wu M, Zhang Y, Zhang X, Li M and Wang G: Mapping the flavor contributing traits on" Fengwei melon"(Cucumis melo) chromosomes using parent resequencing and super bulked-segregant analysis. PLoS One 2016; 11(2): 0148150.
- Zheng XY and Wolff DW: Ethylene production, shelf-life and evidence of RFLP polymorphisms linked to ethylene genes in melon (Cucumis melo). Theoretical and Applied Genetics 2000; 101: 613-24.
- Perin C, Hagen L, De Conto V, Katzir N, Danin-Poleg Y, Portnoy V, Baudracco-Arnas S, Chadoeuf J, Dogimont C and Pitrat M: A reference map of Cucumis melo based on two recombinant inbred line populations. Theoretical and Applied Genetics 2002; 104: 1017-34.
- Burger Y, Paris HS, Cohen R, Katzir N, Tadmor Y, Lewinsohn E and Schaffer AA: 3 Genetic Diversity of Cucumis melo. Horticultural reviews 2010; 36(1): 165-98.
- Luan F, Delannay I and Staub JE: Chinese melon (Cucumis melo) diversity analyses provide strategies for germplasm curation, genetic improvement, and evidentiary support of domestication patterns. Euphytica 2008; 164: 445-61.
- Zhao G, Lian Q, Zhang Z, Fu Q, He Y, Ma S, Ruggieri V, Monforte AJ, Wang P, Julca I and Wang H: A comprehensive genome variation map of melon identifies multiple domestication events and loci influencing agronomic traits. Nature Genetics 2019; 51(11): 1607-15.
- Farcuh M, Copes B, Le-Navenec G, Marroquin J, Jaunet T, Chi-Ham C, Cantu D, Bradford KJ and Van Deynze A: Texture diversity in melon (Cucumis melo): Sensory and physical assessments. Postharvest Biology and Technology 2020; 159: 111024.
- Zhang Y, Fan X, Aierken Y, Ma X, Yi H and Wu M: Genetic diversity of melon landraces (Cucumis melo) in the Xinjiang Uygur autonomous region on the basis of simple sequence repeat markers. Genetic Resources and Crop Evolution 2017; 64: 1023-35.
- Zhao G, Lian Q, Zhang Z, Fu Q, He Y, Ma S, Ruggieri V, Monforte AJ, Wang P, Julca I and Wang H: A comprehensive genome variation map of melon identifies multiple domestication events and loci influencing agronomic traits. Nature Genetics 2019; 51(11): 1607-15.
- Moing A, Allwood JW, Aharoni A, Baker J, Beale MH, Ben-Dor S, Biais B, Brigante F, Burger Y, Deborde C and Erban A: Comparative metabolomics and molecular phylogenetics of melon (Cucumis melo, Cucurbitaceae) biodiversity. Metabolites 2020; 10(3): 121.
- Mallek‐Ayadi S, Bahloul N and Kechaou N: Phytochemical profile, nutraceutical potential and functional properties of Cucumis melo seeds. Journal of the Science of Food and Agriculture 2019; 99(3): 1294-301.
- Ibrahim ME and El-Masry HG: Phenolic content and antioxidant activity of cantaloupe (Cucumis melo cantalupensis) and food application. Int J Nutr Food Sci 2016; 5(1): 16-24.
- Al-Sayed HM and Ahmed AR: Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Annals of Agricultural Sciences 2013; 58(1): 83-95.
- Mallek‐Ayadi S, Bahloul N and Kechaou N: Phytochemical profile, nutraceutical potential and functional properties of Cucumis melo seeds. J of the Science of Food and Agriculture 2019; 99(3): 1294-301.
- Petkova Z and Antova G: Proximate composition of seeds and seed oils from melon (Cucumis melo) cultivated in Bulgaria. Cogent Food & Agriculture 2015; 1(1): 1018779.
- Azhari S, Xu YS, Jiang QX and Xia WS: Physicochemical properties and chemical composition of Seinat (Cucumis melo tibish) seed oil and its antioxidant activity. Grasas y aceites 2014; 65(1).
- Mallek-Ayadi S, Bahloul N and Kechaou N: Chemical composition and bioactive compounds of Cucumis melo seeds: Potential source for new trends of plant oils. Process Safety and Environmental Protection 2018; 113: 68-77.
- Laur LM and Tian L: Provitamin A and vitamin C contents in selected California-grown cantaloupe and honeydew melons and imported melons. Journal of Food Composition and Analysis 2011; 24(2): 194-201.
- Norrizah JS: "β-carotene and antioxidant analysis of three different rockmelon (Cucumis melo) cultivars." 2012); 1846-1852.
- Ismail HI, Chan KW, Mariod AA and Ismail M: Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chemistry 2010; 119(2): 643-7.
- Mehra M, Pasricha V and Gupta RK: Estimation of nutritional, phytochemical and antioxidant activity of seeds of musk melon (Cucumis melo) and water melon (Citrullus lanatus) and nutritional analysis of their respective oils. JPP 2015; 3(6): 98-102.
- Azhari S, Xu YS, Jiang QX and Xia WS: Physicochemical properties and chemical composition of Seinat (Cucumis melo tibish) seed oil and its antioxidant activity. Grasas y Aceites 2014; 65(1).
- Gill NS, Bajwa J, Kunal Dhiman, Sharma P, Sood S, Sharma PD, Singh B and Bali M: "Evaluation of therapeutic potential of traditionally consumed Cucumis melo " 2011.
- Arora R, Kaur M and Gill NS: "Antioxidant activity and pharmacological evaluation of Cucumis melo agrestis methanolic seed extract." 2011; 146-155.
- Siddeeg A, Alsir E, Xu Y, Jiang Q and Xia W: Chemical composition and antibacterial activity of the essential oil isolated from seinat (Cucumis melo Tibish) Seeds. International Journal of Technology Enhancements and Emerging Engineering Research 2014; 2(8): 120-4.
- Gill NS, Bajwa J, Sharma P, Kunal Dhiman, Sood S, Sharma PD, Singh B and Bali M: "Evaluation of antioxidant and antiulcer activity of traditionally consumed Cucumis melo" (2011): 82-89.
- Parmar HS and Kar A: Protective role of Mangifera indica, Cucumis melo and Citrullus vulgaris peel extracts in chemically induced hypothyroidism. Chemico-Biological Interactions 2009; 177(3): 254-8.
- Rasouli H, Parvaneh S, Mahnam A, Rastegari-Pouyani M, Hoseinkhani Z and Mansouri K: Anti-angiogenic potential of trypsin inhibitor purified from Cucumis melo seeds: Homology modeling and molecular docking perspective. Int J of Biological Macromolecules 2017; 96: 118-28.
- Chen L & Kang YH: In-vitro inhibitory effect of oriental melon (Cucumis melo L. var. makuwa Makino) seed on key enzyme linked to type 2diabetes: assessment of anti-diabetic potential of functional food. Journal of Functional Foods 2013; 5(2): 981-986
How to cite this article:
Gaikwad KP, Narkhede MB and Chinchole PP: An overview of Cucumis melo L. physicochemical traits and health-promoting qualities. Int J Pharmacognosy 2024; 11(9): 445-51. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.11(9).445-51.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
445-451
511 KB
974
English
IJP
Kiran P. Gaikwad *, Mahesh B. Narkhede, Jaya P. Ambhore and Chanchal S. Chandak
Department of Pharmacy, Dr. Rajendra Gode College of Pharmacy, Malkapur, Maharashtra, India.
kiranpgaikwad10@gmail.com
06 August 2024
17 September 2024
27 September 2024
10.13040/IJPSR.0975-8232.IJP.11(9).445-51
30 September 2024