AMINO ACID ANALYSIS USING ION-EXCHANGE CHROMATOGRAPHY : A REVIEW
HTML Full TextAMINO ACID ANALYSIS USING ION-EXCHANGE CHROMATOGRAPHY: A REVIEW
Chatrapal Singh * 1, C. S. Sharma 2 and P. R. Kamble 1
Department of Quality Assurance 1, Department of Pharmaceutics Chemistry 2, Bhupal Nobels’ College of Pharmacy, Udaipur - 313001, Rajasthan, India.
ABSTRACT: Amino acids are biologically active substances, and a number of them are essential for living beings. Amino acids are found in living cells as well as in body fluids of higher animals, in amounts, which vary according to the tissue and particular amino acid. Amino acid analysis refers to the methodology used to determine the amino acid composition or content of proteins, peptides, and other pharmaceutical preparations. Proteins and peptides are macromolecules consisting of covalently bonded amino acid residues organized as a linear polymer. The sequence of the amino acids in a protein or peptide determines the properties of the molecule. Proteins are considered large molecules that commonly exist as folded structures with a specific conformation, while peptides are smaller and may consist of only a few amino acids. Amino acid analysis can be used to quantify protein and peptides, to determine the identity of proteins or peptides based on their amino acid composition, to support protein and peptide structure analysis, to evaluate fragmentation strategies for peptide mapping, and to detect atypical amino acids that might be present in a protein or peptide. It is necessary to hydrolyze a protein/peptide to its amino acid constituents before amino acid analysis. The perfect method for the determination of the amino acid composition of pure protein feeds or biological fluids is still the Ion Exchange Column Chromatography (IEC).
| Keywords: |
Ion Exchange Column Chromatography, Amino acids, Determination, Hydrolysis
INTRODUCTION: Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Separation can be selectively achieved by adsorption and release of samples from the matrix. Ion exchange starts with the equilibration of the exchanger using pH and ionic strength. During equilibration, the exchangeable groups are associated with counter-ions.
Once equilibrium is reached, and the sample added the molecules undergo addition and adsorption with an appropriate charge displace the counter ions and bind reversibly to the matrix.
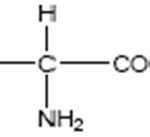
FIG. 1: THE GENERAL STRUCTURAL FORMULA OF AMINO ACIDS (where R* can be H, CH3, C(CH3)2, etc.)
The unbound materials will pass through the column with the void volume. In the third stage, substances are removed from the column by increasing the ionic strength of the eluting buffer 1, 2. Amino acids are biologically active substances, and a number of them are essential for living beings. Amino acids are found in living cells as well as in body fluids of higher animals, in amounts, which vary according to the tissue and particular amino acid 3.
Importance of Amino Acid Analyses: The techniques of amino acid analyses have gained paramount importance in biochemical research during the past two decades. There are three important fields of biochemical investigations in which it is routinely applied:
- The investigation of the structure and composition of proteins particularly in the determination of their amino acid sequence.
- The determination of amino acids in physiological fluids and tissues.
- The determination of the free amino acid composition of foodstuffs to ascertain their food values. Free amino acids can be estimated directly or after separation from the contaminating material by passing through ion exchange columns. Amino acids in proteins and peptides are estimated after a hydrolyzing agent breaks the peptide bonds and they are set free. There are three kinds of hydrolyzing methods, which can be used. Each has advantages in particular cases. Since the hydrolytic procedure is of crucial importance, and the hydrolyzing agent used effects the amino acid composition 3, 4.
Sample Preparation: The correct separation of the samples is the base of the accurate and repeatable analysis of amino acids by automatic IEC. Before the preparation of the samples, the protein content or the approximate content of amino acids should be known for the selection of the optimum weighing of the original sample.
The sample has to be as pure as possible because some of the constituents of the sample can assist to destroy the sensitive amino acids. The volumes of the sample which can be applied to the ion exchange column vary for the different instruments. With refinements in instrumentation, the tendency has been pointing towards a decrease of the sample volume to 50 µl or less.
The preparation of the sample can be divided into two parts depending on the purpose of investigation: releasing the amino acids from protein and Peptides using hydrolysis, and preparation of samples containing free amino acids when the protein and other disturbing substances are removed.
Hydrolysis Method:
Acid Hydrolysis: Dilute H2SO4 (5 to 6 N) was used in many of the earlier investigations on protein structure, but extensive losses of amino acids were reported due to their absorption on precipitated BaSO4.3 This method is generally not employed now. The most usually employed acidic reagent is 6N HCl 4. It has the advantage that it can be removed readily from the hydrolysate; the most serious problem is the loss of tryptophan, whereas threonine, serine, and tyrosine are partly destroyed 5. In this method, methionine and cystine were either partially destroyed or oxidized to methionine sulphone and cysteic acid. Most of these problems arose from the presence of oxygen in the hydrolyzing solution and the presence of the free halogen in the 6N HCl.
The difficulty of incomplete hydrolysis has been overcome by increasing the duration of hydrolysis from 24 h to 72 h 6. Tryptophan has been recovered by using 3N p-Toluene sulphonic acid and a two- percent (2%) protective agent, 3 (2- aminoethyl) indole, as a hydrolyzing agent in the method developed by Liu and Chang. In the most recent procedure for hydrolysis, a double distilled HCl (6N), free from halogen, is used 6.
Alkaline Hydrolysis: Alkaline hydrolysis of protein is of limited use. The destruction of arginine, serine, threonine, cysteine, and cystine precludes its general application 7. It is usually applied only in determining amino acids that are labile to acids, in particular, tryptophan. Tryptophan is destroyed least when hydrolyzes with 4N Ba(OH)2 is carried out at 110 °C for 50 to 70 h 8. The hydrolysates after precipitation of excess of barium are subjected to chromatographic separation of amino acids.
Enzymatic Hydrolysis: To avoid the marked losses of certain amino acids that occur during acid hydrolysis and for the estimation of asparagines and glutamine, enzymatic hydrolysis provides best method 9. The digestion of a protein by papain followed by treatment with the purified protein peptidases and prolidases gave essentially complete hydrolysis of all peptide bonds and liberated tryptophan, glutamine, and asparagines in high yield 9.
Ion Exchange Resins Used For Analysis: Amino acids in a protein hydrolysate can conveniently be separated for qualitative and quantitative analyses by automated Ion Exchange Chromatography. Two general classes of immobile ion exchangers are most frequently used by biochemists 10.
(a) Synthetic resin backbone ion exchangers,
(b) Polysaccharide backbone ion exchangers.
We would like to focus on the ion exchange resins having synthetic backbone only. They are porous and elastic particles, containing synthetic resin backbone, usually of polystyrene type, formed by the copolymerization of styrene and divinyl-benzene. Desired functional groups, such as strongly acidic (-SO3H), strongly basic (-NR3+), weakly acidic (-COOH) and weakly basic (-NH3+) can be introduced by the replacement of styrene with corresponding substituted styrene analog
TABLE 1: POLYSTYRENE TYPE ION EXCHANGERS AND THEIR CHARACTERISTICS
| Type of Exchange | Functional Group | Common Name | pH | Ionic Capacity |
| Weak cation exchange | Carboxymethyl | CM Cellulose Sepharose | 5-9 | 0.09-0.13m mol(Cl-)/ml |
| Strong cation exchange | Sulfopropyl | SP Fast Flow Sepharose | 6-10 | 0.18-0.25m mol(Cl-)/ml |
| Weak anion exchange | Diethylaminoethyl | DEAE Cellulose Sepharose | 5-9 | 0.11-0.16m mol(Cl-)/ml |
| Strong anion exchange | Quaternary Ammonium | Q Sepharose | 2-9 | 0.18-.25m mol(Cl-)/ml |
Ion Exchange Chromatography of Amino Acids: Griessbach 11 was one of the first to separate amino acids with the aid of ion exchange resins. Freedenberg 12 and also Block 13 described methods for the separation of amino acids into groups and also for their separation from non-electrolytes, with ion exchangers. After sample preparation, in most cases, meaning hydrolysis of the protein or preparation of the sample for free amino acid analysis, depending on the amino acids present in the sample, sodium or lithium buffers are prepared for separation of the amino acids by IEC. The eluate from the ion exchange column is passed through in a teflon coil placed in a boiling water bath, or other heating apparatus.
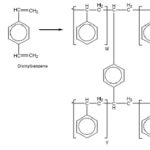
FIG. 2: COPOLYMERIZATION OF STYRENE AND DIVINYLBENZENE MAKES POLYSTYRENE RESINS
Before entering, the column eluent is mixed with reduced ninhydrin reagent, which is dissolved in acetate buffer. The ninhydrin reacts with amino acids forming a dye complex. The absorption is determined in a flow photometer and registered on the chart of a recorder or a computer. The area under the peaks corresponds to the amounts of amino acids present in the sample. The evaluation can be done manually or automatically with an integrator or a computer. The circumstances of the analysis make it possible to quantitate as little as one nano-mol amino acid with a high degree of accuracy.
Adsorption and separation of the amino acids on the ion exchanger depend upon their dissociation constants. Anion exchangers in acid form will absorb all amino acids, and any non-electrolytes such as sugar will be separated from them 14. By using these resins in the neutralized form, i.e. Na+ or NH4+ form, neutral amino acids can be selectively adsorbed and dicarboxylic acids and histamine will not be absorbed. Weakly acidic resins are more suitable for separation of all types of amino acids 14.
The ion exchange takes place on resin, consisting of small spherical beads of polystyrene, reacted with divinylbenzene to achieve the required degrees of cross-linkage between the two polymerized chains of styrene, and sulfonated to provide an electrical charge. The chromatographic column is filled with resins of negative charge, and the amino acids are put on the column at a low pH value (pH=2.2), hence all of them bear a positive charge. In these conditions all of the amino acids will link to the resin, no chromatographic division will occur, and the amino acids are waiting at the beginning of the column for a change in conditions. If the pH and the ionic strength of the elution buffers increase, the isoelectric point of the amino acids will be reached, and the attraction of the ions towards the resin diminishes, and so the amino acids will be eluted from the column 15, 16.
The isoelectric point of an amino acid molecule is defined as the pH value, at which the molecule, in the solution, do not dispose of any charge 15, 16. The low cross-linking resins with 1-4% divinylbenzene have higher permeability, their equilibrium is reached more rapidly, and they are capable of handling larger molecules. The capacity of the resins, because of the swollen volume is smaller, the separation power for certain ions is reduced, and the physical stability of the resin is also less. The low cross-linking resins with 8-16% divinylbenzene have a small pore size, lesser permeability, but it is sufficient for more minor ions, and the swelling is slight 15, 17, 18.
Examining the particle size of the resin, it is advisable, that the smallest possible particle size is the best. The exchange rate increases with decreasing particle size since the diffusion path between the active groups become shorter. Short diffusion values improve the sharpness of the separation and permit to use shorter columns reducing the separation time. Smaller particles have higher mechanical stability which is to be considered very important, because the resin expands and contracts in the column through the continuous changes in pH and concentration during the analysis 19, 20.
The dimension of the separating column is very important as regards to the high-resolution separation between the amino acids. The diameter of the columns nowadays is 1-2 mm, but earlier columns with 5-9 mm diameter were widely used. The larger diameter columns are preparative columns. The separating performance depends in addition to the diameter of the ion exchange particles, on a length factor and the column diameter. It is preferable to keep the column as narrow as possible to have the largest possible number of the theoretical plate number in the column 15, 21.
The flow rate of the eluting buffer on the column is very important, as it determines the time of the analysis. If the flow rate through the column is more than the optimal, the fractions leaving the column become unsymmetrical, leading to tailing, also, the amino acid peaks can overlap. Increasing flow rate leads to a higher back pressure, which is undesirable for safety. The regeneration of the ion exchange column is indispensable after the sufficient number of amino acid analysis. During the regeneration sodium hydroxide or lithium hydroxide is used to wash the impurities from the column and replace the Na+ or Li+ ions used during the analysis. Some authors suggest 0.2-1.0 M, but the optimum concentration seems to be 0.4M for sodium hydroxide and 0.3 for lithium hydroxide.
If cation resins contaminated with heavy metals, proteins or other bigger molecules, the resin have to be removed from the column, treated with 1% EDTA in 2M hydrogen chloride solution for some hours at room temperature, regenerated by boiling the resin in 6M HCl for half an hour, cooled at room temperature, diluted to 3M HCl, filtered and washed with 500 ml two times distilled water. Remove the resin from the filter and suspend in 2M NaOH or LiOH depending on Na or Li system. Boil the resin for some minute, and dilute to the 0.5M base. This resin is ready to fill in the analytical column 17, 18.
Buffer Systems for Separation of the Amino Acids:
Choice of Buffer System: Generally, protein hydrolysates contain most of all 18 amino acids normally found in proteins; they are easily separated with three sodium buffer system. Physiological fluids contain some of all the 40-50 ninhydrin positive compounds present in different physiological mixtures. For this purpose, four or five sodium buffer system is suitable to achieve a satisfactory separation between the ninhydrin-positive compounds. The lithium buffer system is suitable for these purposes, but the application of this system is justified rather in the case that simultaneous separation of aspartic acid, asparagine, glutamic acid, and glutamine is required.
The lithium system is more sensitive to variations than the sodium system. The salts used for making buffers should be at the highest purity. The salts should be dissolved in deionized or carefully distilled water. Not only the ninhydrin positive impurities, but others may cause irregularities in the baseline, for this reason freshly drawn deionized water is preferred. The acidic buffers tend to take up ammonia and other ninhydrin-positive compounds; therefore it is advisable to add the HCl as late as possible to the buffers 17, 18.
Effect on Separation by pH, Temperature, Organic Solvents and Column Flow Rate: The pH of the buffer is very critical for the separation of various amino acids. All of the peaks of amino acids emerge earlier and sharper if the pH is too high, and peaks the chromatograph later if the pH is too low. The cysteine is the most sensitive for the pH, temperature and the concentration of the ions with an opposite charge of the buffer. Cystine should be eluted and completely separated directly after alanine. With increasing pH and temperature the column accelerates the cysteine, thereby shortens its elution time and if the temperature and pH lower, its elution times become longer, and cystine falls behind.
The pH value and temperature must be selected in a way, that cysteine can just be positioned between alanine and valine. The pH change has a greater influence on the cystine movement than a change in temperature. The temperature affects the separation in two different ways: by changing the pH and by altering the affinity of the amino acids to the ion exchange resin.
The separation between threonine and serine can be improved by lowering the temperature, but at the same time the backpressure is increased substantially, and it influences the separation of the glutamic acid. Therefore it is important to have a temperature gradient after the separation of the two hydroxy amino acids. Cystine is also sensitive to temperature, but the pH can easily compensate for any changes in the retention time caused by the temperature.
In the system for hydrolysates, the increase of the temperature from 50 °C to 70 °C or higher is recommended to decrease the time of analysis, but this rise should not take place before the separation of isoleucine and leucine. The optimum temperature for separation of aspartic acid, hydroxyl proline, threonine, serine, asparagine, glutamic acid, and glutamine is 37-38 °C with both sodium or lithium buffer systems, as glutamic acid is particularly sensitive even to minor changes of temperature.
The organic solvent added to the first buffer changes the solubility of the different amino acids. It is particularly the extra -CH3 group of threonine as compared to serine that results in melioration in separation. The most frequently used compounds are methanol, ethanol, propanol, iso-propanol, and methylcellulose. The drawback of these techniques is a slight loss of separation between glycine and alanine and increased back pressure. It is possible to use as much as 25% of organic solvent, but the normally used concentration is between 2% and 5%.
The analysis should be started at a rather low percentage of organic solvent, providing an acceptable separation between threonine and serine, and increases the amounts when the column becomes older, and the peaks slightly broader. The limiting factor should be the separation between glycine and alanine. A steady buffer flow rate is required for successful and reproducible separations of amino acids by IEC. This can be achieved with constant pressure or a constant displacement pump. At most of the analyzers the pumps are pulse-free and feature an even power output and their utilization guarantees conformity of the retention times of individual peaks.
The pressure limit of the pumps is 1 to 8MPa and is controlled by the software. The choice of flow rate is dependent upon the type of resin, the dimensions of the column and the overall design of the instrument, and it varies between models 15-21.
TABLE 2: THE COMPOSITION OF THE SODIUM CITRATE BUFFERS 17
| Buffer | ||||
| 1 | 2 | 3 | 4 | |
| M Na | 0.2 | 0.2 | 0.4 | 1.12 |
| M citrate | 0.066 | 0.066 | 0.066 | 0.066 |
| pH | 2.60 | 3.00 | 4.25 | - |
| Citric acid (g/dm3) | 30 | 30 | 32 | - |
| Sodium citrate (g/dm3) | 19.6 | 19.6 | 19.6 | 19.6 |
TABLE 3: THE COMPOSITION OF THE LITHIUM CITRATE BUFFERS 17, 22
| Buffer | |||||
| 1 | 2 | 3 | 4 | 5 | |
| M Li | 0.18 | 0.20 | 0.35 | 0.33 | 1.20 |
| M citrate | 0.053 | 0.060 | 0.070 | 0.100 | 0.220 |
| pH | 2.90 | 3.10 | 3.35 | 4.05 | 4.65 |
| Citric acid (g/dm3) | 27.26 | 30.07 | 35.17 | 38.48 | 41.65 |
| Lithium citrate (g/dm3) | 14.92 | 16.92 | 19.74 | 28.20 | 62.04 |
| Lithium chloride (g/dm3) | 7.62 | 8.47 | 14.83 | 13.98 | 50.87 |
Detection Systems: The color or fluorescence produced of amino acids varies for different amino acids, and it has to be determined for quantification. It can be made by loading a mixture of amino acids containing the same concentration of each amino acid (including the chosen internal standard) and from the areas of the peaks on the recorder trace calculating each response factor in the used way.
Sometimes an internal standard, absent from the sample, is used for every analysis carried out. For instance, the non-physiological amino acids norleucine or α-amino-β-guanidino-butyric acid may be used. This should be added in a known amount to the sample before any sample pre-treatment. If the amount of the internal standard is known, the concentration of the unknown amino acids can be determined using peak area relationship.
CONCLUSION: Amino acid analysis, using various techniques, it can be stated that although there are recently developed methods of amino acid analyses, such as gas chromatography, which are faster and less expensive, ion exchange method of amino acid analysis is the best despite its being expensive, and will continue to be used for this purpose for years to come because of its reproducibility, recently achieved better speed, and complete automation.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Himmelhoch SR: Chromatography of proteins onion-exchange adsorbents Meth Enzymol 1971; 22:273-286.
- Scopes RK: Ion exchangers-principles, properties and uses. In Protein Purification: Principles and Practice, Springer-Verlag, New York, 1982: 75-101.
- Hugli TE and Moore S: J Biol Chem 1972; 247: 2828.
- Lu TY and Chang YY: J Biol Chem 1971; 246: 2843.
- Davies MG and Thomas AJ: J Sci Food Agri 1973; 24: 1525.
- Tarr GE: Methods of protein micro characterization. Humana Press, Clifton NJ, 1986.
- Moore S and Stein WH: Methods in Enzymology, Academic Press, New York, Vol. 6, 1963.
- Noltman EA, Mahowald TA and Kuby SA: J Biol Chem 1962; 237: 1146.
- Hill RL and Schmidt WR: J Biol Chem 1962; 237: 389.
- West KA and Crabb JW: Techniques in Protein Chemistry III, Academic Press, New York, 1992.
- Griessbach R: Angew Chem 1939; 52: 215.
- Freedenberg K: Organische Chemie, Heidelberg Quelle U. Meyer, 1948.
- Block RJ: Pro Sec Exp Biol Med 1949; 72: 337.
- Nazaki S: Separation Method of Amino Acids, US Patent 53006539, 1994.
- Belitz HD and Grosch W: Food Chemistry, Springer, 1999; 1-992.
- Holm DJ and Peck H: Analytical Biochemistry, Longman, 1998; 91-168.
- Amino acid analyser AAA 400. User manual INGOS, 2002.
- Amino acid analysis. Handbook and application LKB Biochrom, 1982.
- Cooper C, Packer N and Williams K: Amino acids protocols, Humana Press 2001; 1-265.
- Friedman M: Protein nutrition quality of foods and feeds. Assay methods-biological, biochemical and chemical, Marcel Dekker, 1975; 21: 1-626.
- Moughan PJ, Verstegen MWA and Visser-Reyneveld MI: Feed evaluation. Principles and practice, Wageningen Pers, 2000.
- Pickering MV: U.S. Patent, 1981; 4-274-833.
How to cite this article:
Singh C, Sharma CS and Kamble PR: Amino acid analysis using Ion-Exchange Chromatography: a review. Int J Pharmacognosy 2014; 1(12): 756-62. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(12).756-62.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
756-762
573
3030
English
IJP
C. Singh *, C. S. Sharma and P. R. Kamble
Department of Quality Assurance Bhupal Nobel’s College of Pharmacy, Udaipur, Rajasthan, India
chatrapalsingh88@gmail.com
19 April 2014
26 October 2014
15 November 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(12).756-62
01 December 2014