ACUTE AND SUB-ACUTE TOXICITY STUDY OF COMPOUND SIDDHA DRUG LAGU SEENA CHOORANAM FOR THE MANAGEMENT OF SCABIES
HTML Full TextACUTE AND SUB-ACUTE TOXICITY STUDY OF COMPOUND SIDDHA DRUG LAGU SEENA CHOORANAM FOR THE MANAGEMENT OF SCABIES
A. Silambarasan *, S. Gandhimathi and V. Rani
Department of Paediatrics, Govt. Siddha Medical College, Chennai - 600106, Tamil Nadu, India.
ABSTRACT: Herbals are playing a major role in Siddha system of medicine. Herbals contain plant materials as their pharmacologically active components. The aim of the study is to evaluate the acute and sub-acute toxicity of the Siddha herbal preparation Lagu Seena Chooranam (LSC) for the treatment of Scabies in the pediatric age group. For acute studies, different doses of LSC were administered orally to rats once daily for one week. For sub-acute studies, different doses were administered orally to rats once daily for 28 days in various doses at 200, 400 mg/kg of body weight. Detailed hematological, biochemical, necropsy and histopathological evaluation of organs were performed for all animals. Histopathological analysis revealed that spleen, testes, pancreas, lung, liver, brain, heart, stomach, intestine, bone, ovary, and kidney tissues of treated groups did not show any signs of toxicity. No destruction in hepatic, renal, hemopoietic functions was observed throughout the study.
| Keywords: |
Siddha medicine, Lagu seen a Chooranam, Scabies, acute and sub-acute toxicity
INTRODUCTION: Plants are using for the medicinal purpose since from the ancient days. These medicinal plants are described as herbals. Herbals contain plant materials as their pharmacologically active components. These are playing a significant role in Siddha system of medicine. Siddha system is one of the popular traditional medicinal systems in India. Traditional medicinal resources, especially plants have been found to play a major part in managing skin disorders 1. Plants are the only economic source of some well-established and important drugs. Also, they are also the source of chemical intermediates needs for the production of some drugs 2. However, many issues related to a lack of scientific evidence about the efficacy and safety of herbal remedies remains unresolved 3, 4.
Pre-clinical toxicity studies are essential to determine a safe dose for human trial 5. Before the initiation of human clinical trials of novel drugs, the safety of their application is to be proved. Generally, this is accomplished by the implementation of extensive preclinical toxicity experiments to uncover potential poisonous effects of any drug in animals 6.
The present study was conducted to evaluate the acute and sub-acute toxicity of the Siddha drug “Lagu Seena Chooranam (LSC).” The interventional drug “Lagu Seena Chooranam” has been quoted by Agasthiar Vaithiya Pillai Thamizh 7. The drug is chosen for the treatment of Sirangu (Scabies) for the pediatric age group. This report aims to provide vital information about the efficacy and safety of the Siddha drug Lagu Seena Chooranam.
MATERIALS AND METHODS:
SOP of Lagu Seena Chooranam: Paranki pattai (Smilax china), Shivanar vembu verpattai (Indigofera aspalathoides), Sirukurinjan verpattai (Gymnema sylvestre), Thalaisuruli verpattai (Aristolochia indica), Sangankuppi ilai (Clerodendrom inerme), Sangan verpattai (Azima tetracantha), Vellarugu samoolam (Enicostemma axillare), Kaiyanthagarai samoolam (Eclipta prostrate), Sengathari verpattai (Capparis sepiaria). Above mentioned plants were taken equal quantity. It were dried, Purified and made into fine powder 7.
Species: Male and female Wistar rats of age 6 - 8 weeks old.
Environmental Conditions: Air-conditioned rooms, the temperature was between 22 ± 2° C and the illumination cycle set to12 hours light and 12 hours dark.
Accommodation: Standard polypropylene rat cages with stainless steel top grill cleaned paddy husk was used as the bedding material. Animals were housed in groups of three animals of similar sex.
Sanitation: Bedding material and water bottles were changed daily.
Diet and Water: Standard pellet feed was provided. Potable water passed through ad libitum in rat feeding bottles with stainless steel sipper tubes 8.
Acute Toxicity Study in Rats 9:
Procedure: An acute toxicity study was carried out as per OECD guideline (Organization for Economic Co-operation and Development) 423. Healthy female rats weighing 220–240 gm were used for this study. Studied carried out at female rats divided into two groups of 3 animals each under fasting condition (16 h before test animals deprived of food, not for water), signs of toxicity were observed for every one hour for first 24 h and every day for about 14 days from the beginning of the study. All animals were observed daily for clinical signs. The time of onset, intensity, and duration of these symptoms, if any, were recorded.
Observation: Animals were observed for possible signs of toxicity related to CNS, ANS, and CVS.
Acute Toxicity Study Grouping:
Group I: Control Group: 3 female rats
Group II: Treatment group: 3 female rats
Dose: 2000 mg/kg
Route: Oral route (Single dose administration)
Sub-Acute Toxicity Study in Rats 10:
Procedure: A sub-acute toxicity study was carried out as per OECD guideline (Organization for Economic Co-operation and Development, Guideline-407. Animals were allowed an acclimatization period of 7 days to laboratory conditions before the initiation of treatment. Three rats of same-sex were housed per cage. Eighteen rats (09 male and 09 healthy female animals) were randomly divided into three groups of 6 animals.
Treatment groups were dose daily for 28 days. Control group animal left untreated; Animal belongs to group II treated with low dose of the test drug 200 mg/kg and Group III treated with high dose of the test drug 400 mg/kg, per oral by gastric intubation technique. All animals were observed daily for clinical signs of toxicity. The time of onset, intensity, and duration of the symptoms, if any, were recorded. Body weight, food intake, water intake and other general behavior of the animals will be monitored and recorded once in a week for the entire duration of the study.
Sub- Acute toxicity study Grouping:
Group I: Control Group: 6 rats (3 male and 3 female)
Group II: Treatment group: 6 rats(3 male and 3 female) - Low dose 200mg/kg
Group III: Treatment group: 6 rats(3 male and 3 female)- High dose 400mg/kg
Dose: Low dose 200mg/kg and high dose 400 mg/kg
Route: Oral route (repeated dose administration)
Hematological and Biochemical Investigations: 11 Blood was collected through retro-orbital sinus from all the animals of different groups on the 29th day. The blood was collected in tubes containing Heparin/EDTA as an anticoagulant. Animals have fasted overnight before the blood collection. Hematological and biochemical parameters were determined using Auto analyzer using standard kits, and the data are provided.
Necropsy: All animals were sacrificed by cervical dislocation on the 29th day. Necropsy of all animals were carried out, and the weights of the vital organs were recorded (heart, liver, kidneys, and brain).
Histopathology: During necropsy the target organs viz., heart, liver, kidneys, and brain were collected and preserved in 10 % neutral formalin buffer for the histopathological evaluation. The organs from control and treated animals were preserved in 10 % neutral formalin buffer for histopathological examination.
RESULTS AND DISCUSSION:
Acute Toxicity Study: Acute toxicity effect of the test drug was estimated by close observation of animals for about 24 h after single dose administration of the test drug, and it was observed that there are no significant signs of CNS related toxicity like convulsion, locomotion, muscle strength and ANS related toxicity like salivation, lacrimation, etc. was observed in the treatment group.
At the end of the study period, all animals were sacrificed, and the organs were isolated and observed for change in structural morphology. There is no significant change in the organ necropsy of the animals treated with the test drug. It shows that the test drug hasn’t produced any internal hemorrhage or organ related toxicity.
TABLE 1: PARAMETER CHECKED
| Group | Day |
| Body weight | Normal |
| Assessments of posture | Normal |
| Signs of Convulsion
Limb paralysis |
The absence of sign (-) |
| Body tone | Normal |
| Lacrimation | Absence |
| Salivation | Very mild |
| Change in skin color | No significant color change |
| Piloerection | Not observed |
| Defecation | Regular Solid consistency |
| Sensitivity response | Normal |
| Locomotion | Normal |
| Muscle grip ness | Normal |
| Rearing | Normal |
| Urination/Color | Slightly turbid |
TABLE 2: EFFECT OF TEST DRUG-ON MORTALITY RATE OF THE STUDY ANIMALS ON ACUTE TOXICITY STUDY
| Treatment | Mortality observed for the duration of 1- 14 days |
| Group I - Control | NIL |
| Group Ii- Treatment | NIL |
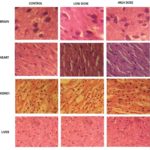
Sub - Acute Toxicity Study: Sub-acute toxicity for the given test drug was carried out as per the OECD guideline 407 by repeated dose administration of the test drug in animals, and further animals were closely monitored for the emergence of toxicity. Since, there were no significant adverse effects on the hematological, biochemical and histopathological parameters it may be concluded that the test drug at both the dose level of 200 mg/kg and 400 mg/kg may be considered as relatively safe, as it did not cause either mortality or produce severe toxicological effects on selected body organs, biochemical indices and hematological and histopathological markers of rats during the sub-acute periods of study Fig. 1.
TABLE 3: EFFECT OF TEST DRUG-ON MORTALITY RATE OF THE STUDY ANIMALS ON SUB-ACUTE TOXICITY STUDY
| Treatment | Mortality observed for the duration of 1- 28 days |
| Group I - Control | NIL |
| Group Ii- Low Dose | NIL |
| Group Iii- High Dose | NIL |
TABLE 4: EFFECT OF TEST DRUG-ON BODY WEIGHT AND FOOD CONSUMPTION
| Control | Food (g/day/rat) | Body weight (g) |
| Mean | 24.67 | 234.5 |
| Std. Deviation | 2.422 | 2.429 |
| Std. Error | 0.9888 | 0.9916 |
| LOW DOSE | Food (g/day/rat) | Body weight (g) |
| Mean | 23.67 | 234 |
| Std. Deviation | 3.386 | 2 |
| Std. Error | 1.382 | 0.8165 |
| HIGH DOSE | Food (g/day/rat) | Body weight (g) |
| Mean | 22.17 | 232.7 |
| Std. Deviation | 1.472 | 2.805 |
| Std. Error | 0.6009 | 1.145 |
TABLE 5: EFFECT OF TEST DRUG ON HEMATOLOGICAL AND BIOCHEMICAL ANALYSIS
| Control | Total red cells count (×106 µl) | Total WBC count (×103 µl) | Platelet count (×103 µl) | Packed cell volume (%) | MCV (fl) | MCH (pg) | MCHC (g/dl) | Blood sugar ® (mg/dl) | BUN (mg/dl) |
| Mean | 6.667 | 9.333 | 546.3 | 56 | 62.67 | 31.67 | 50 | 88.17 | 19.5 |
| Std. Deviation | 1.506 | 2.733 | 41.52 | 9.529 | 5.785 | 4.412 | 2.28 | 5.707 | 2.881 |
| Std. Error | 0.6146 | 1.116 | 16.95 | 3.89 | 2.362 | 1.801 | 0.9309 | 2.33 | 1.176 |
| LOW DOSE | Total red cells count (×106 µl) | Total WBC count (×103 µl) | Platelet count (×103 µl) | Packed cell volume (%) | MCV (fl) | MCH (pg) | MCHC (g/dl) | Blood sugar ® (mg/dl) | BUN (mg/dl) |
| Mean | 7.667 | 9.167 | 584.8 | 49.17 | 57.5 | 29.33 | 44.67 | 71.83 | 14.17 |
| Std. Deviation | 0.5164 | 0.4082 | 20.42 | 2.714 | 4.68 | 5.715 | 1.633 | 1.602 | 2.994 |
| Std. Error | 0.2108 | 0.1667 | 8.336 | 1.108 | 1.91 | 2.333 | 0.6667 | 0.654 | 1.222 |
| HIGH DOSE | Total red cells count (×106 µl) | Total WBC count (×103 µl) | Platelet count (×103 µl) | Packed cell volume (%) | MCV (fl) | MCH (pg) | MCHC (g/dl) | Blood sugar ® (mg/dl) | BUN (mg/dl) |
| Mean | 6 | 9.5 | 502.7 | 55.17 | 61.33 | 31.67 | 41.33 | 83.33 | 20.33 |
| Std. Deviation | 1.095 | 1.975 | 15.45 | 1.602 | 5.086 | 3.141 | 5.785 | 3.724 | 3.141 |
| Std. Error | 0.4472 | 0.8062 | 6.307 | 0.654 | 2.076 | 1.282 | 2.362 | 1.52 | 1.282 |
TABLE 6: EFFECT OF TEST DRUG ON SERUM CREATININE AND LIPID PROFILE
| Control | Serum creatinine (mg/dl) | Serum total cholesterol (mg/dl) | Serum triglycerides level (mg/dl) | Serum HDL cholesterol (mg/dl) | Serum LDL cholesterol (mg/dl) | Serum VLDL cholesterol (mg/dl) | Serum total protein (g/dl) |
| Mean | 0.7333 | 104.3 | 45 | 23.5 | 54.83 | 40.67 | 6.3 |
| Std. Deviation | 0.1506 | 7.394 | 2.098 | 1.871 | 3.371 | 3.386 | 2.825 |
| Std. Error | 0.06146 | 3.018 | 0.8563 | 0.7638 | 1.376 | 1.382 | 1.153 |
| Low dose | Serum creatinine (mg/dl) | Serum total cholesterol (mg/dl) | Serum triglycerides level (mg/dl) | Serum HDL cholesterol (mg/dl) | Serum LDL cholesterol (mg/dl) | Serum VLDL cholesterol (mg/dl) | Serum total protein (g/dl) |
| Mean | 1.033 | 102.8 | 49.33 | 20.67 | 51.33 | 36.33 | 5.517 |
| Std. Deviation | 0.1862 | 6.853 | 5.428 | 2.875 | 2.338 | 3.777 | 0.5115 |
| Std. Error | 0.07601 | 2.798 | 2.216 | 1.174 | 0.9545 | 1.542 | 0.2088 |
| High dose | Serum creatinine (mg/dl) | Serum total cholesterol (mg/dl) | Serum triglycerides level (mg/dl) | Serum HDL cholesterol (mg/dl) | Serum LDL cholesterol (mg/dl) | Serum VLDL cholesterol (mg/dl) | Serum total protein (g/dl) |
| Mean | 1.133 | 104.3 | 54.17 | 25.17 | 55.83 | 39 | 5.333 |
| Std. Deviation | 0.1862 | 2.805 | 1.835 | 2.317 | 2.041 | 2.966 | 1.506 |
| Std. Error | 0.07601 | 1.145 | 0.7491 | 0.9458 | 0.8333 | 1.211 | 0.6146 |
TABLE 7: EFFECT OF TEST DRUG ON ALBUMIN AND LIVER ENZYMES ANALYSIS
| Control | Serum albumin (g/dl) | SGOT (AST) (IU/ml) | SGPT (ALT) (IU/L) |
| Mean | 4.7 | 126.2 | 65.67 |
| Std. Deviation | 1.934 | 4.401 | 3.077 |
| Std. Error | 0.7895 | 1.797 | 1.256 |
| LOW DOSE | Serum albumin (g/dl) | SGOT (AST) (IU/ml) | SGPT (ALT) (IU/L) |
| Mean | 3.083 | 147.2 | 74.67 |
| Std. Deviation | 0.343 | 9.867 | 1.506 |
| Std. Error | 0.14 | 4.028 | 0.6146 |
| HIGH DOSE | Serum albumin (g/dl) | SGOT (AST) (IU/ml) | SGPT (ALT) (IU/L) |
| Mean | 4.667 | 116.2 | 70 |
| Std. Deviation | 0.5164 | 1.835 | 6.753 |
| Std. Error | 0.2108 | 0.7491 | 2.757 |
TABLE 8: EFFECT OF TEST DRUG ON BLOOD CELL COUNT
| Control | HB (g/dl) | Neutrophils (%) | Lymphocytes (%) | Eosinophils (%) | Monocytes (%) | Basophils (%) |
| Mean | 15.5 | 75.17 | 36.5 | 1.85 | 0.6667 | 0.5 |
| Std. Deviation | 1.761 | 2.927 | 3.391 | 0.3782 | 0.2066 | 0.5477 |
| Std. Error | 0.7188 | 1.195 | 1.384 | 0.1544 | 0.08433 | 0.2236 |
| LOW DOSE | HB (g/dl) | Neutrophils (%) | lymphocytes (%) | eosinophils (%) | monocytes (%) | basophils (%) |
| Mean | 16.67 | 76.5 | 34.83 | 1.483 | 0.9333 | 0.1667 |
| Std. Deviation | 1.211 | 1.643 | 2.317 | 0.3189 | 0.1033 | 0.4082 |
| Std. Error | 0.4944 | 0.6708 | 0.9458 | 0.1302 | 0.04216 | 0.1667 |
| HIGH DOSE | HB (g/dl) | Neutrophils (%) | lymphocytes (%) | eosinophils (%) | monocytes (%) | basophils (%) |
| Mean | 15.5 | 73.5 | 36.17 | 1.333 | 0.5667 | 0.3333 |
| Std. Deviation | 0.5477 | 2.881 | 6.08 | 0.2422 | 0.1966 | 0.5164 |
| Std. Error | 0.2236 | 1.176 | 2.482 | 0.09888 | 0.08028 | 0.2108 |
TABLE 9: EFFECT OF TEST DRUG-ON ORGAN MORPHOLOGY
| Grouping | Kidney | Liver | Heart | Lungs | Spleen | Pancreas | Brain | Ovaries | Testes |
| Group I – Control | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Group II- Low Dose | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Group III- High Dose | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
FIG. 1
CONCLUSION: The present exploration demonstrates that the doses consumed in the traditional medicine, i.e., LSC may be considered as relatively safe, as it does not cause either mortality or produce severe toxicological effects on selected body organs, biochemical incite and hematological markers of rats during the acute and sub-acute periods of study.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Abbasi AM, Khan MA, Ahmad M, Zafar M, Jahan S and Sultana S: Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J Ethnopharmacol 2010, 128: 322-335.
- Samraj K, Thillaivanan S and Parthiban P: A review of beneficial effects of medicinal plants on skin and skin Diseases. IJPRBS 2014; 3(1): 93-106
- Shekelle PG, Morton SC, Suttorp MJ, Buscemi N and Friesen C: Challenges in systematic reviews of complementary and alternative medicine topics. Ann Intern Med 2005; 142: 1042-1047
- Patil RB, Vora SR and Pillai MM: Protective effect of Spermatogenic activity of Withania somnifera (Ashwagandha) in galactose stressed mice, Annals of Biological Research, 2012; 3(8): 4159-4165. (http://scholarsresearchlibrary.com/archive.html).
- Anoop A, Jagadeesan M and Subramanium S: Toxicological studies on Linga Chendooram-I, a Siddha drug, Indian J Pharma Sci 2002; 64(1): 53.
- Farzamfar B: Sub-chronic toxicity study of a novel herbal-based formulation (Semelil) on dogs, DARU 2008; 16(1). (http://journals.tums.ac.ir/ on Friday, May 24, 2013).
- Anonymous, Agasthiyar Vaithiya Pillai Thamizh, Thamarainoolagam, Vadapalani, Chennai 1999; 72.
- Benjamin MN: Outline of Veterinary Clinical Pathology. University Press, IOWA, USA 1978: 229-232.
- Organization for Economic Cooperation Development (OECD) Guideline, 425, 2000. Guideline Document on Acute Oral Toxicity. Environmental Health and Safety Monograph Series on Testing and Assessment No. 24.
- OECD (testing guideline, 407). Repeat dose 28 days oral toxicity study in rodents; In Guidance document for the development of OECD guideline for testing of chemicals Environmental monographs; 1995: No 76; http//www.oecd.ord/document/30/0.2340,en??2649-34377-19166381111, 00html.
- Ringler DH and Dabich L: Hematology and Clinical Biochemistry. In: The Laboratory Rat. Baker, J., J.R. Lindsey and S.H. Weisbroth (Eds.), Academic Press London 1979; 1: 105-118.
How to cite this article:
Silambarasan A, Gandhimathi S and Rani V: Acute and sub-acute toxicity study of compound Siddha drug lagu seena chooranam for the management of scabies. Int J Pharmacognosy 2015; 2(10): 497-02. doi: 10.13040/IJPSR.0975-8232.2(10).497-02.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
497-502
658
1222
English
IJP
A. Silambarasan *, S. Gandhimathi and V. Rani
Department of Paediatrics, Govt. Siddha Medical College, Chennai, Tamil Nadu, India.
simbudoctor@gmail.com
24 August 2015
25 September 2015
20 October 2015
10.13040/IJPSR.0975-8232.IJP.2(10).497-02
31 October 2015