A REVIEW ON LIQUISOLID TECHNOLOGY TO ENHANCE DRUG DISSOLUTION
HTML Full TextA REVIEW ON LIQUISOLID TECHNOLOGY TO ENHANCE DRUG DISSOLUTION
Rama Brahma Reddy *, K. Malleswari, H. G. Diwakar, D. Kadhar Basha and K. John Abhi
Nalanda Institute of Pharmaceutical Sciences, Siddharth Nagar, Kantepudi, Sattenapalli, Guntur, Andhra Pradesh, India.
ABSTRACT: Dissolution of drug and its release from the dosage form have basic impact on bioavailability. The solubility of a medication determines its bioavailability. As new medications are developed, solubility becomes a significant concern for the pharmaceutical industries. About 40% of recently developed medications fit into the poor water soluble or water insoluble classifications. Generally speaking, weakly water-soluble medications have an aqueous solubility of less than 100μg/ml. There are numerous ways to improve the solubility of poorly soluble medications, and one particularly effective method is the use of liquisolid compacts. A unique idea for a novel drug delivery system (NDDS) that can speed up the rate at which water-insoluble medications dissolve is the liquisolid compact system. The basis of this liquisolid method is the dissolution of the insoluble drug in a non-volatile solvent, followed by the mixing of drug-loaded solutions with suitable carriers and coating materials to produce powders that flow and compress into acceptable levels. These free-flowing powders are compressed to make tablets or filled in capsules.
Keywords: Liquisolid, Poor water solubility, Carrier material, Coating material, Powdered solution technology
INTRODUCTION: Oral route is the most desirable and preferred method of administering therapeutic agents for their systemic effects. In addition, the oral route medication is generally considered as the first avenue investigated in the discovery a development of new chemical entities and pharmaceutical formulations mainly because of patient acceptance, convenience in administration and cost-effective manufacturing process. For many drug substances, conventional immediate release formulation Provide clinically effective therapy while maintaining the required balance of pharmacokinetic and pharmacodynamic profile with an acceptable level of safety to the patient.
Thus, one of the major challenges to drug development today is poor solubility, as an estimated 40% of all newly developed drugs are poorly soluble or insoluble in water:
- Nowadays the synthesis of poorly soluble drugs increasing steadily. Therapeutic effectiveness of a drug depends upon the bioavailability which is dependent on the solubility and dissolution rate of drug molecules. Solubility is one of the important Parameter to achieve desired concentration of drug in systemic circulation for pharmacological response to be shown.
- There are several methods for enhancing dissolution rate of poorly water-soluble drugs including.
- Reducing particle size to increase surface area, thus increasing dissolution rate of drug. Solubilization in surfactant systems. Formation of water-soluble complexes. Drug dramatization such as a strong electrolyte salt forms that usually have higher dissolution rate. Manipulation of solid state of drug substance to improve drug dissolution, i.e. by decreasing crystallinity of drug substance through formation of solid solutions 1, 2.
Historical Development: Historically, liquisolid compacts are descendants of ‘powdered solutions’, an older technique which was based on the conversion of a solution of a drug in a nonvolatile solvent into a dry-looking, nonadherent powder by mainly adsorbing the liquid onto silicas of large specific surfaces. Such preparations, however, have been investigated for their dissolution profiles while being in a powder dispersion form and not as compressed entities, simply because they could not be compressed into tablets. In later studies on powdered solutions, compression enhancers such as microcrystalline cellulose were added in such dispersions in order to increase the compressibility of the systems. In these studies, however, large quantities of silicas were still being used, and the flow and compression properties of the products were never validated and standardized to industrial specifications and requirements. Liquisolid compacts, on the other hand, are acceptably flowing and compressible powdered forms of liquid medications, and have industrial application. In addition, the term ‘liquid medication’ does not only imply drug solutions, as in powdered solutions, but also drug suspensions, emulsions, or liquid oily drugs. Therefore, in contrast to ‘powdered solutions’, the term ‘liquisolid compacts’ is more general and it may encompass four different formulation systems namely:
- Powdered drug solutions
- Powdered drug suspensions
- Powdered drug emulsions
- Powdered liquid drugs Furthermore, the earlier term ‘powdered solutions’ seems to be inadequate even in describing the original systems, since it has not been proven that the drug remains in solution in the liquid vehicle after its deposition on the extremely large powder surfaces of silica used. The new ‘liquisolid’ technique may be applied to formulate liquid medications [i.e., oily liquid drugs and solutions, suspensions or emulsions of water-insoluble solid drugs carried in nonvolatile liquid vehicles] into powders suitable for table ting or encapsulation. Simple blending of such liquid medications with calculated quantities of a powder substrate consisting of certain excipients referred to as the carrier and coating powder materials can yield dry-looking, nonadherent, free-flowing, and readily compressible powders.
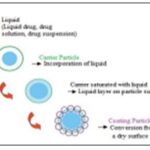
Concept: When the drug dissolved in the liquid vehicle is incorporated into a carrier material which has a porous surface and closely matted fibers in its interior as cellulose, both absorption and adsorption take place; i.e. the liquid initially absorbed in the interior of the particles is captured by its internal structure, and after the saturation of this process, adsorption of the liquid onto the internal and external surfaces of the porous carrier particles occur. Then, the coating material having high adsorptive properties and large specific surface area gives the liquisolid system the desirable flow characteristics Fig. 1 3.
FIG. 1: STEP IN FORMULATION OF LIQUISOLID
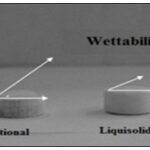
The wettability of the compacts by the dissolution media is one of the proposed mechanisms for explaining the enhanced dissolution rate from the liquisolid compacts.
Nonvolatile solvent present in the liquisolid system facilitates wetting of drug particles by decreasing interfacial tension between dissolution medium and tablet surface. Fig. 2 shows lower contact angle of liquisolid compacts than the conventional tablets and thus improved wettability.
FIG. 2: COMPARISON OF WETTABILITY BETWEEN CONVENTIONAL TABLET AND LIQUISOLID COMPACTS
The Characteristics of Liquid Vehicle Include:
- Orally safe
- Chemically Inert
- Not highly viscous
- Preferably water-miscible organic solvent systems with a high boiling point.
- Examples; glycerine, propylene glycol, or liquid polyethylene glycols
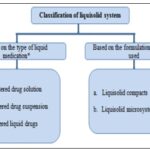
Classification of Liquisolid System: Based on the type of liquid medication contained therein, liquisolid systems may be classified into three subgroups
- Powdered drug solutions
- Powdered drug suspensions
- Powdered liquid drugs
The first two may be produced from the conversion of drug solutions or (e.g. prednisolone solution in propylene glycol13 drug suspensions (e.g. gemfibrozil suspension in polysorbate 8032, and the latter from the formulation of liquid drugs (e.g. clofibrate, liquid vitamins, etc.) into liquisolid systems.
Based on the formulation technique used, liquisolid systems may be classified into two categories 4.
- Liquisolid compacts
- Liquisolid Microsystems
Method of Preparations: The liquisolid tablet preparation method involves, first a mathematically calculated amount of pure drug weighed and dissolved in the suitable amount of solvent in a molecularly dispersed state. For attaining good flow properties trial and error methods were used i.e. changing the carrier: coating material ratio from 50:1 to 5:1 ratio according to new mathematical model expressions proposed by Liao. This liquid medication is poured on the suitable amount of carrier material. The liquid medication is absorbed into the carrier material internally and externally and then a suitable disintegrant was added to this material. Finally, coating material was added for dry looking, adherent to the carrier material for achieving good compression properties.
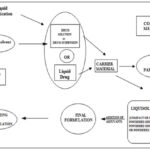
Liquid medication is incorporated into carrier material which has a porous surface and closely matted fibers in its interior as cellulose. Both absorption and adsorption take place, i.e. the liquid absorbed into the interior of the particles is captured by its internal structure and after saturation of this process, adsorption of the liquid onto the internal and external surface of the porous carrier particles occurs. Excipients possessing a fine and highly adsorptive particles such as various types of amorphous silicon dioxide (silica) are most suitable for this step. Before compression or encapsulation, various ingredients such as lubricants disintegrants or Polymers, and binders (as shown in Fig. 3), may be mixed with the finished liquisolid systems to produce liquisolid compacts in the dosage form of tablets or capsules.
Examples of Some Drugs that can be Incorporated into Liquisolid Systems:
- Chlorpheniramine
- Digoxin
- Nifedipine
- Clofibrate
- Gemfibrozil
- Etoposide
- Carbamazepine
- Hydrochlorothiazide
- Methyclothiazide
- Spironolactone
- Hydrocortisone
- Piroxicam
- Indomethacin
- Ibuprofen
Determination of Drug in Different Non-volatile Solvents: These are carried by preparing saturated solutions of the drug in non-volatile solvents and analyzing them spectrophotometrically. Saturated solutions are prepared by adding an excess of the drug to vehicles and shaking them on a shaker for the specific time period under steady vibration. After this, the solutions are filtered and analyzed spectrophotometrically 5.
FIG. 3:
Pre-formulations Studies:
- Determination solubility of drug in different non-volatile solvents.
- Determination of angle of slide.
- Determination of flowable liquid retention potential (Φ value).
- Calculation of liquid load factor (LF).
- Liquisolid compressibility test (LSC).
Solubility Studies: Solubility studies are carried out by preparing saturated solutions of drug in non-volatile solvent and analyzing them spectrophotometrically. Saturated solutions are prepared by adding excess of drug to non-volatile solvent and shaking them on shaker for specific time period under constant vibration. After this, the solutions are filtered and analyzed spectrophotometrically.
Determination of Angle of Slide: Angle of slide is used as a measure of the flow properties of powders. Determination of angle of slide is done by weighing the required amount of carrier material and placed at one end of a metal plate with a polished surface. The end is gradually raised till the plate becomes angular to the horizontal at which powder is about to slide. This angle is known as angle of slide. Angle of 33º is regarded as optimum.
Determination of Flowable Liquid Retention Potential (Φ Value): The term "flowable liquid-retential potential" (Φ-value) of a powder material describes its ability to retain a specific amount of liquid while maintaining good flow properties. The Φ-value is defined as the maximum weight of liquid that can be retained per unit weight of the powder material in order to produce an acceptably flowing liquid/powder admixture.
The Φ values are calculated according to equation
Φ value = weight of liquid / weight of solid
Calculation of Liquid Load Factor (LF): Different concentrations of non-volatile solvents are taken and the drug is dissolved. Such liquid medication is added to the carrier coating material admixture and blended. Using equation (2) drug loading factors are determined and used for calculating the amounts of carrier and coating materials in each formulation.
LF = weight of liquid medication / weight of carrier materia
Liquisolid Compressibility Test (LSC): Liquisolid compressibility test is used to determine Φ values and involves steps such as preparing carrier coating material admixture systems, preparing several uniform liquid or powder admixtures, compressing each liquid or powder admixtures to tablets, assessing average hardness, determination of average liquid content of crushed tablets, as well as determining plasticity, sponge index and Φ value and LF 6.
Evaluation of Liquisolid Granules:
Flow Behaviour: The flowability of a powder is of critical importance in the production of pharmaceutical dosage forms in order to reduce high dose variations.Angle of repose, Carr’s index and Hausner’s ratio were used in order to ensure the flow properties of the liquisolid systems.
Angle of Repose: Angle of repose, Carr’s index and Hausner’s ratio were used in order to ensure the flow properties of the liquisolid systems. This is the maximum angle possible between the surface of a pile of powder and the horizontal plane.10 gm of powder was allowed to flow by funnel from 4 cm of height from the base. The height of pile and diameter of the base was measured and calculate the angle of repose by the following the formula 7.
tan θ = h/r
θ = tan-1 h/r
Where = angle of repose,
h = Height of the heap,
r = Radius of the heap.
Bulk Density: An accurately weighed quantity of powder, which was previously passed through sieve # 40 [USP] and carefully poured into a graduated cylinder. Then after pouring the powder into the graduated cylinder, the powder bed was made uniform without disturbing. Then the volume was measured directly from the graduation marks on the cylinder as ml.
The volume measured was called as the bulk volume and the bulk density is calculated by following formula.
Bulk density = Weight of powder/Bulk volume
Tapped Density: After measuring the bulk volume, the same measuring cylinder was set into tap density apparatus. The tap density apparatus was set to 300 taps drop per minute and operated for 500 taps.
Volume was noted as [Va] and again tapped for 750 times and volume was noted as [Vb]. If the difference between Va and Vb not greater than 2% then Vb is to consider as final tapped volume. The tapped density is calculated by the following formula
Tapped density = Weight of powder/Tapped Volume
Carr’s Index [Compressibility Index]: It is one of the most important parameters to characteristic the nature of powders and granules. It can be calculated from the following equation:
Carr’s index = Tapped Density - Bulk density / Tapped density × 100
Hausner’s Ratio: Hausner’s ratio is an important character to determine the flow property of powder and granules. This can be calculated by the following formula:
Hausner’s ratio = Tapped density / Bulk density
Evaluation of liquisolid tablets. Weight variation Weight variation was measured by weighing 20 Tablets and average weight was found and percentage weight variation of the individual tablet should fall within specified limits in terms of percentage deviation from the mean. Thickness the thickness of the tablet was measured by vernier caliper. Hardness it is a measure of the mechanical strength of a tablet using a hardness tester [Monsanto hardness tester].
The mechanical strength of a tablet is associated with the resistance of a tablet to fracture or attrition. Friability it was determined using Roche friabilator, the percentage loss in tablet weight before and after 100 revolutions of tablets was calculated and taken as a measure for friability. Disintegration time the time necessary to disintegrate 3 tablets of each tablet formulation was determined using a disintegration tester.
In-vitro dissolution studies it is carried out as given in particular monograph of the drugs tablet formulation 8.
Evaluation of Liquisolid Tablets:
Weight Variation: Twenty tablets were randomly selected from each batch and individually weighed. The average weight and standard deviation three batches were calculated.
It passes the test for weight variation test if not more than two of the individual tablet weights deviate from the average weight by more than the allowed percentage deviation and none deviate by more than twice the percentage shown. It was calculated on an electronic weighing balance (Smirnova I, Suttiruengwong S et al 2004) 9.
Thickness: The thickness of liquisolid tablets was determined by using Digital micrometer. Ten individual tablets from each batch were used and the results averaged.
Hardness: The hardness of the tablets was determined by using Monsanto hardness tester. Five individual tablets from each batch were and results averaged.
Friability: The friability values of the tablets were determined using a Roche-type friabilator. Accurately weighed six tablets were placed in Roche friabilator and rotated at 25 rpm for 4 min. Percentage friability was calculated using the following equation.
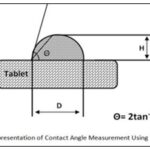
Contact Angle: Measurement For assessment of wettability, contact angle of liquisolid tablets is measured according to the imaging method. The commonly used method is to measure contact angle directly for a drop of liquid resting on a plane surface of solid, the so-called imaging method.
A saturated solution of drug in dissolution media is prepared and a drop of this solution is put on the surface of tablets. The contact angles are calculated by measuring the height and the diameter of sphere drop on the tablet. Fig. 4 represents the measurement of contact angle using imaging method 10.
FIG. 4: SCHEMATIC REPRESENTATION OF CONTACT ANGLE MEASUREMENT USING IMAGING METHOD
Advantages of Liquisolid Systems:
- Number of water-insoluble solid drugs can be formulated into liquisolid systems.
- Can be applied to formulate liquid medications such as oily liquid drugs.
- Better availability of an orally administered water insoluble drug.
- Lower production cost than that of soft gelatin capsules
- Production of liquisolid systems is similar to that of conventional tablets.
- Can be used for formulation of liquid oily drugs
- Exhibits enhanced in-vitro and in-vivo drug release as compared to commercial counterparts, including soft gelatin capsule preparations.
- Can be used in controlled drug delivery.
- Drug release can be modified using suitable formulation ingredients
- Drug can be molecularly dispersed in the formulation.
- Capability of industrial production is also possible. Enhanced bioavailability can be obtained as compared to conventional tablets.
Disadvantages of Liquisolid System:
- The liquisolid systems have low drug loading capacities and they require high solubility of drug in non-volatile liquid vehicles.
- It requires more efficient excipients which have higher adsorption capacities which provide faster drug release with a smaller tablet size to improve liquisolid formulations.
- To maintain acceptable flowability and compatibility for liquisolid powder formulation high levels of carrier and coating materials are require and that in turn will increases the weight of each tablet above 1 gm which is very difficult to swallow.
Applications: Enhancement of Solubility and Dissolution. An effective technique for increasing the bioavailability of water-insoluble medications is liquisolid compact technology. A number of water insoluble medications have been combined into liquisolid compacts by dissolving them in various non-volatile solvents. Several medications have been effectively combined into liquisolid compacts, according to literature. Liquisolid compositions yield rapid release rates. These work well for both liquid lipophilic medicines and solid medications that are insoluble in water. This approach has been used to generate sustained release of water-soluble medicines, such as propranolol hydrochloride. Flowability and Compressibility; Design of Controlled Release Tablets. Improvement of Bioavailability 11.
Limitations: Not applicable for the formulation of high dose insoluble drugs. If more amount of carrier is added to produce free flowing powder, the tablet weight increases to more than one gram which is difficult to swallow. Acceptable compression properties may not be achieved since during compression liquid drug may be squeezed out of the liquid-solid tablet resulting in tablets of unsatisfactory hardness. Introduction of this method on industrial scale and to overcome the problems of mixing small quantities of viscous liquid solutions onto large amounts of carrier material may not be feasible 12.
CONCLUSION: The addition of disintegrants may further accelerate drug release from liquisolid compacts. The liquisolid technology may also be used for the preparation of sustained release formulations with zero order release pattern. Thus, a constant plasma level will be reached, which is maintained throughout the dosing interval. For sustained release liquisolid compacts, the selection and the concentration of the excipients such as a liquid vehicle, retarding agent (matrix forming material) as well as carrier and coating material play an important role
ACKNOWLEDGMENT: The authors are expressing sincere thanks to the principal and management of Nalanda institute of pharmaceutical sciences, Sidharth Nagar, Kantepudi (V), Sattenapalli (M), Guntur dist-522438, AP, for supporting the work.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Doijad RC, Phatan AB, Gaikwad SS, Baraskar SS, Pawar NB and Maske VD: “Liquisolid: A novel Technique for dissolution enhancement of poorly soluble drugs.” International Journal of Liquisolid 2012; 3(1): 735-749.
- Nagabandi Kumar Vijay, Ramarao T and Jayaveera KN: Liquisolid compacts: A novel approach to enhance bioavailability of poorly soluble drugs.” International Journal of Liquisolid 2011; 1(3): 89-102.
- Yousef Javadzadeh, Baharak Jafari Navimipour and Ali Nokhodchi: Liquisolid Technique for Dissolution Rate Enhancement of a High Dose Water-Insoluble Drug (carbamazepine). Inter J of Pharma 2007; 341: 26–34.
- Spireas S and Bolton S: Sustainedrelease Liquisolid Compacts. In: 25th International Symposium on Controlled Release of Bioactive Materials Nevada USA 1998; 138–139.
- Nazzal S and Khan MA: Controlled release of a self-emulsifying formulation from a tablet dosage form: Stability assessment and optimization of some processing parameters. International J of Liquisolid 2006; 315: 110-21
- Javadzadeh Y, Musaalrezaei L and Nokhodchi A: Liquisolid technique as a New Approach to Sustain Propranolol Hydrochloride Release Form Tablet Matrices. International Journal Pharm 2008; 362: 102-108.
- Sambasiva RA and Naga AT: Liquisolid technology: an overview. International journal Res Pharm Biomed Sci 2011; 2: 401-9.
- Satheeshbabu N, Gowthamarajan K, Gayathri R and Saravanan T: “Liquisolid: a novel technique to enhance bioavailability.” J. of Pharmacy Research (JPR) 2011; 4(1): 181-185
- Vajir S: Enhancement of dissolution rate of poorly water-soluble diclofenac sodium by liquisolid technique. Int J Pharm Chem Sci 2012; 1: 989-1000. Edition Vallabh Prakashan New Delhi; 2001.
- Patric JS: Martin’s physical pharmacy and pharmaceutical sciences. 5th ed. Lippincott Williams and Wilkins 2003.
- Chuahan PV, Patel HK, Patel BA, Patel KN, Patel PA. Liquisolid technique for enhancement of dissolution rate of ibuprofen. Int J Pharm Res Scholars 2012; 1: 268-80.
- Baby DA and Saroj S. M Sabitha: Mechanism of solubility of liquisolid formulation in non-volatile solvent: A review. International Journal Pharm Pharma Sci 2012; 4: 710-15.
How to cite this article:
Reddy RB, Malleswari K, Diwakar HG, Basha DK and Abhi KJ: A review on liquisolid technology to enhance drug dissolution. Int J Pharmacognosy 2024; 11(8): 406-13. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.11(8).406-13.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
406-413
897 KB
710
English
IJP
Rama Brahma Reddy *, K. Malleswari, H. G. Diwakar, D. Kadhar Basha and K. John Abhi
Nalanda Institute of Pharmaceutical Sciences, Siddharth Nagar, Kantepudi, Sattenapalli, Guntur, Andhra Pradesh, India.
malleswarikunchala105@gmail.com
15 July 2024
23 August 2024
28 August 2024
10.13040/IJPSR.0975-8232.IJP.11(8).406-13
31 August 2024