A REVIEW ON A MIRACLE PLANT ANNONA GLABRA LINN
HTML Full TextA REVIEW ON A MIRACLE PLANT ANNONA GLABRA LINN
R. Sinchana *, T. Tamizh Mani, T. Pavithra and L. Shiju
Department of Pharmacognosy, Bharathi College of Pharmacy, Bharathinagara, Mandya, Karnataka, India.
ABSTRACT: Plants serve humans as primary sources for food, shelter, and medicines. So, understanding the plant's uses in treating diseases is very important for leading a healthier life. Our ancestors have used various plants as medicines, and there is a need to provide scientific evidence for the same. Annona glabra is one of the plants natives to Florida in the United States, the Caribbean, and central and west Africa. It is an invasive species in Sri Lanka and Australia, and it is also distributed in India. It is commonly called pond apple, alligator apple, or bob wood. These plants contain mostly flavonoids, glycosides, saponins, tannins, steroids, acidic compounds, and anthraquinones. Not much scientific support was given to the folklore claims of the plant, and some of its traditional uses have been investigated, including anti-leishmanial activity, anti-microbial activity, anti-cancer activity, anti-inflammatory activity, burn-healing properties, larvicidal efficacy, wine production, and anti-oxidant activity. This article is a review of research done on the plant Annona glabra. As a part of it, the taxonomy, common names, vernacular names, description, distribution, phytochemicals, and pharmacological activities have been discussed.
Keywords: Annonaceae family, Annona, Pond apple, Phytochemicals, Pharmacological activities, Traditional medicine
INTRODUCTION: Nature has been one of the main sources for the traditional medicine for thousands of years, and large number of modern drugs have been isolated from natural sources 1. Plant-derived natural products have extremely high potential to developed as medicines. Traditional herbal medicine and its preparation are widely used in the developing and developed countries due to their natural origin and lesser side effects 2. Utilizing natural products is considered to have safety, efficacy and quality 3.
In medicinal history, so far, we have come across many medicinal plants used by our ancestors as their primary source for the treatment of various ailments. In consideration of their medicinal uses and phytochemicals, they were widely used in the traditional system of medicine like Ayurveda, Unani and Siddha, each of which had its own medicinal uses. Some of them are scientifically proven, and some are still to be prove 4.
One such plant is Annona glabra, belonging to the genus Annona and the family Annonaceae. It is a very large family of plants, comprising about 120 genera and more than 2000 species. These seem to be one of the least chemically as well as pharmacologically known families compared with other families. This family has economic importance due to its edible fruit and seed oils. Flowers and seeds of certain Annonaceae plants are used in soap preparation, edible oil preparation, and perfumery products. Many members of this family are used in folk medicine to treat various types of tumours and cancers 5.
Annona glabra L., popularly known as Pond Apple, is a tropical fruit tree in the family Annonaceae. It is a natural introduction to the mangroves of southern Kerala and has widely spread along the backwaters. A. glabra is a small-woody tree that grows up to 3–12 m hight 1. A. glabra is made up of small plants (shrubs or trees), is fruitful, has a wide geographic distribution, and is found on the banks of lakes and rivers 6. The members of this family are rich sources of secondary metabolites with high biological activities. Phytochemical compounds of medicinal plants have received more attention in recent years due to their potential role in preventing many human diseases. Many active compounds have been found in A. glabra, mainly flavonoids, glycosides, saponins, tannins, steroids, acidic compounds, and anthraquinones. Recently, a novel class of bioactive compounds called annonaceous acetogenins has aroused tremendous interest 1. Annona plants have several scientifically proven pharmacological effects, such as anticancer, antidiabetic, antidiarrhea, antiulcer, antimalarial, anti-inflammatory, antioxidant, antileishmanial, antibacterial, antifungal, antidepressant, anticonvulsant, antinociceptive, anti-acetyl cholinesterase and dengue vector control activity 3.
Plant Biography 7:
FIG. 1: ANNONA GLABRA PLANT
FIG. 2: ANNONA GLABRA FLOWER
FIG. 3: ANNONA GLABRA FRUIT
FIG. 4: ANNONA GLABRA FRUIT
Taxonomical Classification:
Scientific name: Annona glabra Linn.
Taxonomy:
Kingdom: Plantae
Phylum: Tracheophyta
Subphylum: Angiospermae
Class: Magnoliopsida – Dicotyledons
Order: Magnoliales
Family: Annonaceae
Genus: Annona
Species: Annona glabra
Botanical name: Annona glabra Linn.
Synonyms 8:
- Annona australis
- Annona palustris
- Annona laurifolia
- Annona peruviana
- Annona chrysocarpa
- Annona uliginosa
- Guanabanus palustris
- Asimina arborea
Common names: Pond apple, alligator apple, monkey apple, swamp apple, cow apple, corkwood, bob wood, mangrove Annona 9.
Vernacular Names:
| English: | Pond apple, alligator apple, bob wood, corkwood, cow apple, mangrove Annona, monkey apple |
| Malayalam: | Kattathi, Kattu-Aatha, and Kadalatha |
| Germany: | Alligatorapfel, Alligator-Binnbaum, Annone |
| Netherlands: | Zuurzak |
| Brazil: | Araticum bravo, Araticum do brejo, Araticum-caca, Araticupana |
| China: | Yuan Hua Fan Li Zhi, Niu xin guo |
| France: | Anone des marais, Bois flot, Cachiman cochon, and Corossol des narais |
| Spanish: | Anona Lisa, Anon Liso, Anon de Puerco, and Anonillo Cabuye |
| Japanese: | Pondo appuru |
| Portuguese: | Jaca de pobre, Araticum do brejo, and Araticurana |
Description 10, 11, 12: A. Glabra is a semi-deciduous tree, usually growing up to 3–15 m in height. Usually it has a single bole, with a swollen base when young or narrowly buttressed when mature. Stems are grey with prominent lenticels. The leaves are arranged alternately and are oblong-epithelial, with acute or shortly acuminate tips. They measure between 7 and 13cm in length and up to 6cm in width. The upper surface is light to dark green, while the underside is paler, with a prominent midrib and a distinctive small fold where the leaf blade joins the leaf stalk. Flowers are short-lived and rarely noticed, 2-3 cm in diameter, pale-yellow to cream with three leathery outer petals and three smaller inner petals; pedicel curved, expanded distally; sepals 4.5 mm long, 9 mm broad, apiculate; outer petals valvate, ovate-cordate, cream coloured with a crimson spot at base within, 2.5–3.0 cm long, 2.0–2.5 cm broad; inner petals sub imbricate, shortly clawed, 2.0–2.5 cm long and 1.5–1.7 cm broad, whitish outside, bright-red to dark-crimson within; stigmas sticky, deciduous. When ripened, the fruit changes its colour from green to either yellow or orange, displaying either a spherical or elongated form with a size ranging from 5 to 15cm in diameter. Its appearance resembles that of a smooth-skinned custard apple, and its pulp tends to be rather dry with a distinct pungent-aromatic scent, containing 100–200 light-brown seeds, each 1.5 cm long and 1 cm broad.
| Origin | Florida, the Bahamas, the Caribbean, Central and South America, and West Africa |
| Distribution | coastal areas of northern and central Queensland, cook pastoral district, and North Kennedy and South Kennedy pastoral districts; it is also known to be naturalized in tropical Asia and on several Pacific islands. |
| Habitat | This species prefers wetter tropical and sub-tropical habitats. It generally grows in freshwater and brackish swamps, along creeks and rivers, in rainforests and along rainforest margins, in coastal environs, and along roadsides. |
| U.S.D.A. Zone | 10A-12B (30°F Minimum) |
| Plant Type | Large shrub to medium-sized tree |
| Growth Rate | Moderate |
| Typical Dimensions | 30-40’ tall x 10-20’ wide |
| Reproduction and dispersal | Reproduces by seed and may also produce suckers from damaged roots and trunks. Plants have ability to spread through suckering, lead to the eventual formation of dense thickets. |
| Leaf Persistence | Deciduous, semi-deciduous |
| Leaf Type | Simple |
| Nutritional Requirements | Low |
| Flowering Months | Abundant in spring, but can be year-round. |
| Salt Tolerance | Moderate |
| Light Requirements | Medium, high |
| Soil Requirements | Wide |
| Environmental Concerns | Low |
| Major Potential Pests | Fruit, root |
| Drought Tolerance | Medium |
| Propagation | Seeds |
| Human Hazards | None |
Ethnomedical uses: The A. glabra fruit is edible and can be made into jam. In the Maldives, it is a popular ingredient in fresh fruit drinks. The crushed seed was cooked with coconut oil and applied to get rid of head lice in older days. A. glabra is used in traditional medicines against several ailments, such as fever, constipation, ulcers, and tumours, including cancer. It is a kind of survival food 13. The leaves and young stems, sometimes combined with the leaves and stems of Passiflora foetida, are boiled to make a tea, which is drunk to destroy worms and nematodes. The bark and leaves, combined with the bark and leaves of Annona squamosa, are used as sedatives and cardiotonic infusions. Annonamuricata grafted on A. glabra rootstock receives a dwarfing effect. The wood is used to make bottle caps, oars, and as a substitute for cork in fishing nets. Seeds and leaves are insecticidal, leaves placed in hen nests kill lice on the fowl. Auseful fibre is obtained from the bark. It is sometimes used locally 14.
Phytochemicals: 28 pure compounds were isolated from the fresh fruit of the A. glabra, including 19 kauranediterpenoids,1)16α-hydro-19-acetoxy-ent-kauran - 17 - al, 2) 16β - hydro - ent - kauran - 17-oicacid15,3)16α-hydro-ent-kauran-17-oic acid16, 4)19-nor-ent-kauran-4α-ol-17-oic acid16, 5)16α-hydro-19-ol-ent-kauran-17-oic acid16, 6)ent-kauran-16-en-19-oic acid15,16, 7)16α-hydroxy-ent-kauran-19-oic acid16, 8)16α,17-dihydroxy-ent-kauran-19-oic acid17, 9)16β,17-dihydroxy-ent-kauran-19-oic acid16, 10)16α-hydro-ent-kauran-17,19-dioic acid16, 11)16β-hydroxy-17-acetoxy-ent-kauran-19-oic acid15, 12)16β-hydro-17-hydroxy -ent-kauran-19-al17,13)16α-hydro-17-hydroxy-ent-kauran-19-al18, 14)16β,17-dihydroxy-ent-kauran-19-al17, 15)16α-hydro-19-al-ent-kauran-17-oic acid16, 16)16α-hydro-17-acetoxy-ent-kauran-19-al17, 17)16α-hydro-19-acetoxy-ent-kauran-17-oic acid15, 18) ent-kaur-15-ent-19-Oic acid19, and 19)ent-kaur-15-en-17-ol-19-oic acid16; Four acetogenins, 20) annomontacin20, 21) annonacin21,22) isoannonacinone22, and 23) squamocin23; four steroids, 24) β-sitosterol24, 25)stigmasterol24, 26) β-sitosteryl-D-glucoside24, and 27) stigmasteryl-D-glucoside24; and one oxoaporphine, liriodenine24.
TABLE 1: ETHYL ACETATE EXTRACT OF ANNONA GLABRA FRUIT: CHEMICAL COMPOSITION 25
| Sl. no. | Compound name |
| 1 | Cyclotetradecane |
| 2 | 1-Butene |
| 3 | n-Tridecan-1-ol |
| 4 | (-)-Spathulenol |
| 5 | 1-Hexadecene |
| 6 | Benzene, (1-butyloctyl)- |
| 7 | 3-(4-isopropylphenyl)-2-methylpropionaldehyde |
| 8 | Aromadendrene oxide-(1) |
| 9 | 1H-Cycloprop [e] azulen-7-ol ،decahydro-1،1،7-trimethyl-4-methylene- ، [1ar- (1aα ،4aα ،7β ،7aβ ،7bα)] - |
| 10 | 1-Cyclohexene-1-methanol, 2,6,6-tetramethyl |
| 11 | Isoaromadendrene epoxide |
| 12 | 9-Eicosene, (E)- |
| 13 | 7,10-Pentadecadiynoic acid |
| 14 | Benzene, (1-methylundecyl)- |
| 15 | Cedren-13-ol ،8- |
| 16 | (7,7-dimethyl-1,4-dioxo-2,3,4,5,6,7-hexahydro-1H-inden-2-yl) acetic acid |
| 17 | 3,5-Decadiyne, 2,2-dimethyl- |
| 18 | 3-Isopropyl-6,7-dimethyltricyclo[4.4.0.0(2,8)]decane-9,10-diol |
| 19 | Aromadendrene oxide-(2) |
| 20 | 5,7-Dioxatetracyclo [7.4.0.0(3,10)0. (4,8)]tridecane,2-methylene-11-(1-methy lethyl)-1,6,6-trimethyl |
| 21 | Caryophyllene oxide |
| 22 | Hexadecanoic acid, methyl ester (CAS) |
| 23 | Cis-Z-a-Bisabolene epoxide |
| 24 | 9,12,15-docosatetraenoic acid, methyl ester |
| 25 | 9,12-Octadecadienoic Acid (Z, Z)-, methyl ester |
| 26 | 3-Eicosene, (E)- |
| 27 | Methyl stearate |
| 28 | 9-Octadecenoic acid (Z), - methyl ester (CAS) |
| 29 | Cycloeicosane |
| 30 | Podocarp-7-en-3-one, 13β-methyl-13-vinyl- |
| 31 | n-Tetracosanol-1 |
| 32 | Biformene |
| 33 | n-Propyl5,8,11,14,17-eicosapenta enoate |
| 34 | Androstan-17-ol, 2,3-epoxy, -(2à,3à,5à,17á)- |
| 35 | Kaur-16-en-18-oic acid, methyl ester, (4β)- |
| 36 | 6á- Hydroxytestosterone |
| 37 | Dihydro-isosteviol methyl ester |
| 38 | Kauran-18-al, 17-(acetyloxy)-, (4á)- |
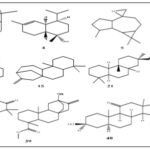
FIG. 5: CHEMICAL STRUCTURES OF THE ETHYL ACETATE EXTRACT OF SOME SELECTED IDENTIFIED COMPOUNDS
TABLE 2: METHANOLIC EXTRACT OF A. GLABRA FRUIT: CHEMICAL COMPOSITION
| S. no. | Compound Name |
| 1 | (-)-Spathulenol |
| 2 | 1,2,3,4,4a,5,6,8a-octahyd ro-4a,8-dimethyl-2-(2-propenyl)- |
| 3 | 2,5-Octadecadiynoicacid,methylester |
| 4 | tau-Cadinol |
| 5 | Aromadendrenepoxide |
| 6 | Ledeneoxide |
| 7 | Caryophylleneoxide |
| 8 | Pentadecanoicacid,methylester |
| 9 | 9-Hexadecenoicacid,methylester,(Z)- |
| 10 | Hexadecanoicacid,methylester |
| 11 | Octadecanoicacidmethylester |
| 12 | Androsta-1,4-dien-3-one17-hydroxy-17-methyl, -, (17à)- |
| 13 | Calarene |
| 14 | Heneicosanoicacid,methylester |
| 15 | Kaur-16-ene، (8β،13β) |
| 16 | 9,12-octadecadienoic acid, methyl ester |
| 17 | 9-Octadecenoic acid (Z), methyl ester (CAS) |
| 18 | Methylstearate |
| 19 | Doconexent |
| 20 | Methyl7,10,13,16,19-docosapentaenoate |
| 21 | Dihydrorimuene |
| 22 | Naphthalene, decahydro-1,1,4a-trimeth yl-6-methylene-5-(3-methyl-2,4-pentadienyl)-, [4aS- (4aà,5à,8aá)] - |
| 23 | Podocarp-7-en-3-one,13β-methyl-13-vinyl- |
| 24 | Oxiraneundecanoic acid, 3-pentyl ester, methyl ester, cis- |
| 25 | Octadecanoicacid,9,10-dihydroxy-,methylester(CAS) |
| 26 | 6-Acetylbenzo[b]naphtho[2,3-e]- [1,4]-dioxin |
| 27 | 13,16-Octadecadiynoicacid,methylester |
| 28 | 6,9,12,15-docosatetraenoic acid, methyl ester |
| 29 | Bicyclo[2.2.1] heptan-2-ol, 2-allyl-1,7,7-trimethyl- |
| 30 | [17-(14-C]-18-hydroxyaphidicol-16-ene |
| 31 | 10,13-eicosadienoic acid, methyl ester |
| 32 | Androstan-3-one17-(acetyloxy)-, (5à,17á)- |
| 33 | Dihydroisopimaric acid methyl ester |
| 34 | Trans-Geranylgeraniol |
| 35 | 1,2,3,3a,4,5,6,8,9,9a,10,10 a-dodecahydro-7-(1-Methylethyl)-1,9a-dimethyl -4- methylene |
| 36 | Kauren-18-ol, acetate, (4á)-(CAS) |
| 37 | Atis-16-ene, (5á,8à,9á,10à,12à) |
| 38 | Dihydro-isosteviol methyl ester |
| 39 | Isosteviol methyl ester |
| 40 | Androstane-6,17-dione,3-hydroxy-, (3á,5à)- |
| 41 | cis-5,8,11,14,17-Eicosapentaenoic acid |
FIG. 6: CHEMICAL STRUCTURES OF THE METHANOLIC EXTRACT OF SOME SELECTED IDENTIFIED COMPOUNDS
TABLE 3: N-BUTANE EXTRACT OF A. GLABRA FRUIT: CHEMICAL COMPOSITION
| S. no. | Compound Name |
| 1 | Tridecanol |
| 2 | (-)-Spathulenol |
| 3 | 6,9,12-octadecatrienoic acid, methyl ester |
| 4 | Soaromadendreneepoxide |
| 5 | Ambrosin |
| 6 | Ledeneoxide-(II) |
| 7 | Alloaromadendrenoxid-(1) |
| 8 | (E)-4-(5',5'-Epoxymethano-1',2',2'-trimethyl-6'-oxo-1'-cyclohexyl)-3-buten-2-one |
| 9 | 4a,7-Methano-4aH-naphth[1,8a-b]oxirene,octahydro-4,4,8,8-tetramethyl |
| 10 | Isoaromadendreneepoxide |
| 11 | Aromadendreneoxide-(2) |
| 12 | (Z)-9-Tetracosene-1,24-diol |
| 13 | 9,12,15-Octadecatrienoicacid |
| 14 | Pregn-4-ene-1,20-dione, 12-hydroxy-16,17-dimethyl- |
| 15 | Hexadecanoicacid,methylester |
| 16 | 11-Octadecenalspectrumdisagrees |
| 17 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5trienyl] cyclohex-1-e-n-1-carboxaldehyde |
| 18 | 9,12-Octadecadienoicacid,methylester,(E, E) |
| 19 | 9-Octadecenoicacid(Z)-,methylester |
| 20 | Octadecanoicacid, methylester |
| 21 | Methyl6-cis,9-cis,11-trans-octadecatrienoate |
| 22 | Estran-3-one, 17-hydroxy (5à, 17á) - |
| 23 | (5à, 17á): Androstan-2-one, (5à) |
| 24 | Androstane-3,11-diol,(3á,5à,11á)- |
| 25 | 5à,14á-Androstane,16à,17à-epoxy- |
| 26 | Podocarp-7-en-3-one,13á-methyl-13-vinyl- |
| 27 | Androstan-3-one,17-(acetyloxy)-,(5α,17β)- |
| 28 | Preg-4-en-3-one,17à-hydroxy-17á-cyano- |
| 29 | Kaur-16-en-19-ol |
| 30 | Cyclooctenone, dimer (CAS) |
| 31 | 1-Phenanthrenecarboxyli c acid, 7-ethenyl-1,2,3,4,4a,4b,5, 6,7,8,10,10a-dodecahydr o-1,4a, 7-trimethyl-methyl ester, [1R-(1à,4aá,4bà,7à,10aà)] |
| 32 | 17-Pentatriacontene |
| 33 | 1-Heptatriacotanol |
| 34 | Ergost-22-en-3-ol,(3á,5à,22E,24R)- |
| 35 | Diisooctyl-phthalate |
| 36 | Isosteviolmethylester |
| 38 | Dihydro-isosteviolmethylester |
| 39 | 1-Naphthalenepropanol, à-ethyldecahydro-5-(hydroxymethyl)-à,5,8a-trimet hyl-2-methylene |
FIG. 7: CHEMICAL STRUCTURES OF THE N-BUTANE EXTRACT OF SOME SELECTED IDENTIFIED COMPOUNDS
TABLE 4: PHYTOCHEMICALS ISOLATED FROM THE LEAVES OF A. GLABRA 3
| Sl. no. | Compound Name |
| 1 | Glabracin A |
| 2 | Glabracin B |
| 3 | Glacins A, Glacins B |
| 4 | Javoricin |
| 5 | Bullatanocin |
| 6 | (-) -(6aS,7R) -7-hydroxyactinodaphnine |
| 7 | (-)-actinodaphnine |
| 8 | (-)-anolobine |
| 9 | (-)-asimilobine |
| 10 | (-)-pallidine |
| 11 | (-)-N-methylactinodaphnine |
| 12 | (-)-roemeroline |
| 13 | (+)-1S,2S-reticuline N-oxide |
| 14 | (+)-boldine |
| 15 | (+)-magnoflorine |
| 16 | (+)-norisodomesticine |
| 17 | (+)-reticuline |
| 18 | (+)-stepharine3- |
| 19 | 3-O-α-L-arabinopyranoside |
| 20 | Liriodenine |
| 21 | Quercetin, Quercetin-3-O-β-D-galactopyranoside |
Phytochemicals Present in the Aqueous Leaf Extract 13: Flavonoids, glycolipids, alkaloids, aromatic hydrocarbons, phenols, sugars, steroids, terpenes. The oil samples predominantly contain mono- and sesquiterpenoids.
TABLE 5: EIGHTEEN COMPOUNDS WERE ISOLATED FROM THE HEXANE EXTRACT OF THE SEED OF A. GLABRA 26
| Sl. no. | Compound Name |
| 1 | Bullatencin |
| 2 | Glabrencin A |
| 3 | Glabrencin B |
| 4 | Uvariamicin- |
| 5 | Uvariamicin- II |
| 6 | Uvariamicin- III |
| 7 | Eticulatain-l |
| 8 | Desacetyluvaricin |
| 9 | 4-deoxyasimicin |
| 10 | Bullatacin |
| 11 | Asimicin |
| 12 | Squamocin |
| 13 | Motrilin |
| 14 | Cherimolin-2 |
| 15 | Palmitic amide |
| 16 | Stigmasterol |
| 17 | Arachidic amide |
| 18 | Stearic amide |
Pharmacological Activities:
Anti-leishmanial Activity: Leishmaniasis is mainly caused by the protozoan parasites of the genus Leishmania and is one of the most neglected tropical diseases, and characterized by the formation of skin ulcers. Despite the global prevalence of numerous cases, the treatment of the disease remains a topic of extensive discussion and is still inadequately understood, despite the considerable attention it has received. In-vitro evaluation of the different Annona species showed antileishmanial activity.
Alkaloids are the substances that show leishmanicidal activity in Annona glabra. Among the alkaloids isolated from the genus species that have already demonstrated anti-Leishmania activity are coronaridine, O-methylarmepavine, 18-methoxycoronaridine, and liriodenine. In addition, the acetogenins corosolone and anonacinone were also promising as leishmanicide. Among kaurenoic acids, terpenes showed activity against the Human Immunodeficiency Virus (HIV) and Trypanosoma cruzi), besides showing antimicrobial activity 27.
Anti-cancer Activity: Recently one of the bioactive compounds called acetogenin has aroused considerable interest. Annonaceous acetogenins from the annonaceae family are a very important source for future antitumor drugs. The acetogenin fractions found in present in A. glabra leaves were detected using Kedde’s reagent, and they were isolated through column chromatography 7. It has long been used in traditional medicine as an anti-cancer agent.
The various parts of the A. glabra cytotoxic study reveal its potential use as an anticancer agent. Previous reports showed that treatment of extract in human leukemia cell lines resulted in reduced mitochondrial fragmentation, reduced ATP content, reduced oxidative stress, and induced apoptosis.
The Ethanolic extract of the A. glabra leaves showed the presence of annoglacins A and B, and it suppressed the proliferation of pancreatic carcinoma and human breast cell lines, and it exerted more pronounced antitumor activity than adriamycin. Icariside D2 is one of the anticancer bioactive compounds; it alters the expression of apoptosis-related proteins decreases the phosphorylation of AKT in HL-60 cells. Studies performed on the methanolic extract of A. glabra seeds on bio-assay guided fraction led to the isolation of the components annonin I, squamocin-C, and squamocin-D.
Among them, annonin I exert higher activity against lung carcinoma A-549, breast adenocarcinoma, and breast adenocarcinoma. Annoglabasin H, a component isolated from the fruits of A. glabra, it showed significant cytotoxic activity in LU-1, MCF-7, SK-Me12, and KB, with IC50 values ranging from 3.7 to 4.6 μM. Some of the reports also showed that A. glabra extract showed cytotoxicity to drug sensitive (CEM) and multidrug-resistant leukemia (CEM/VLB) cell lines.
The seed extract induces necrosis and apoptosis in both sensitive and resistant leukemia cells in a concentration-dependent manner; it also enhances the action of cyclin kinase inhibitors and leads to the arrest of cells at the G0/G1 phase. The cytotoxic activity observed in the hexane extract of the stem bark of A. glabra was associated with the isolation of Kaur-16-en-19-oic acid from a fractionation, indicating its potential therapeutic applications. Annomontacin is one of the anticancer compounds that causes variations in mitochondrial transmembrane and induces apoptosis in the human liver cancer cell line (Hep G2) 28. Cunabic acid and entkauran-19-al-17-oic acid, two diterpenoid compounds isolated from A. glabra, can obviously inhibit the proliferation of the HLC cell line SMMC-7721. The mechanism is correlated with the induction of cell apoptosis by down-regulating the gene expression of thebcl-2 gene and up-regulating that of the bax gene 29.
Anti-inflammatory Activity: Inflammation is a normal and instant response by living tissue to any type of injury. If the inflammation process occurs repeatedly or continuously, it leads to several diseases like rheumatoid arthritis, inflammatory bowel disease, asthma, psoriasis, atherosclerosis, and some cancers. Inflammation, mainly mediated by secretory phospholipase A2(SPLA2S), is known to regulate the arachidonic acid pathway by which pro-inflammatory mediators are released. A. glabra plant showed the presence of several bioactive compounds, like alkaloids, flavonoids, tannins, steroids, terpenoids, glycosides, and phenols, and these could be responsible for its various medicinal properties. To evaluate the mechanism of action of anti-inflammatory activity of the acetone extract of the A. glabra leaves, SPLA2 was subjected to inhibition. The extract showed a good inhibition zone. The synergistic action of the potent active principles of A. glabra leaves may be the reason to inhibit the SPLA2 enzyme to a greater extent 30.
Anti-Microbial Activity: Indian herbal medicines have served as important sources of medicines for the prevention and treatment of many diseases, including microbial infections. The increasing prevalence of multidrug resistance has greatly reduced the available treatment options for antibiotics worldwide, underscoring the critical importance of monitoring bacterial resistance to these drugs. Traditional herbal medicine usage in refined or crude form is very helpful in the treatment of microbial infections with two advantages, i.e., the cure is made by minimizing the chances of microbes becoming resistant. Herbal medicines are very useful in not producing major side effects when compared to antibiotics.
Therefore, in the previous research, A. glabra plant material was tested for its antibacterial activities on the selected strains of bacteria namely, Bacillus cereus, Pseudomonas aeruginosa and Shigella flexneri. These activities were compared with standard antibiotic, namely broad-spectrum antibiotics, ampicillin, and penicillin. The Antimicrobial activity assessment followed the conventional technique of diffusion disc plates on agar. Subsequently, the minimum inhibitory concentration (MIC) was determined by employing the dilution method. Results clearly shown that A. glabra has antimicrobial properties. The plant extract showed more activity than that observed by broad spectrum antibiotic activities. The standard ampicillin and penicillin had MIC values varying between 0.244 mg/mL and 0.488 mg/mL. The results revealed that the antimicrobial potency of the extract of A. glabra exhibits superior antimicrobial efficacy compared to conventional antibiotics. Throughout history, herbs have been recognized for their diverse antimicrobial properties 31.
Ethyl acetate fraction (EAF) was obtained from the leaf hydroalcoholic extract of A. glabra. EAF has bactericidal activity against different strains of Pseudomonas aeruginosa. The viability of P. aeruginosa was observed to be influenced by both time and concentration in the ethyl acetate fraction. Upon testing various subfractions of the ethyl acetate fraction, it was found that subfraction 32-33(SF32-33) exhibited the highest efficacy against P. aeruginosa, displaying a time and concentration-dependent effect. The examination of SF32-33 showed a high content of flavonoids. When incubating P. aeruginosa with this active sub-fraction, ATCC 27983 induces an endothermic reaction. This reaction coincided with an observable increase in electric charge, indicating a strong affinity of SF32-33 compounds for bacterial cell walls. Collectively, flavonoids in the A. glabra are useful for treating infections caused by P. aeruginosa 32. A. glabra fruit extracts showed noticeable in-vitro antimicrobial activity against some tested microbes, including Escherichia coli, Staphylococcus aureus, Aspergillus niger, and Candida albicans. Further, it provides evidence that the antimicrobial activity of the tested extracts could be due to the co-activity between their major and minor constituents. These extracts could be considered an effective therapy to treat various infectious diseases. Therefore, A. glabra fruit could be used as a very good source of naturally occurring antimicrobial remedies 25.
Wood Protection against Termites: A. glabra L. belongs to the family of Annonaceae. A. glabra seed extract contains bioactive substance that is toxic to some organisms, however, the effectiveness to control wood degrading termites has not yet been scientifically reported. Some research analyzes the efficacy of A. glabra seed extract to wood degrading termites. The process of Seed extraction involved the utilization of both n-hexane and ethyl acetate as solvents. This method enabled the retrieval of desired compounds from the seeds efficiently. The extract of A. glabra by paper disc test showed toxic to Coptotermes curvignathus (subterranean termites) and Cryptotermes cynocephalus (dry-wood termites). If the extract concentration (up to 63%) is higher resulted in a higher termite mortality (up to 100%) and lower weight loss of paper sample (less than 1%). When compare to n-hexane extract of the A. glabra seeds, ethyl acetate extract has a better toxicity effect against subterranean termites and dry wood termites 33.
Burn Healing Properties: Burn wounds are the most common accidental injuries. Not only do burn injuries disfigure the skin, but they can also cause severe consequences for body function. These burn injuries can be healed completely with minimal scarring. The whole process of wound healing is a dynamic and intricate process which involves an ordered cascade of events to restore the integrity of damaged tissue. The burn healing process involves different overlapping phases and processes, including haemostasis, inflammation, proliferation, tissue remodelling, and formation of granulation tissue with angiogenesis.
An array of cytokines and growth factors also contribute to the interaction between epidermal and dermal cells, the extra cellular matrix, controlled angiogenesis and plasma-derived proteins in order to facilitate a wound repair process. The phytoconstituents that were detected in the ethanol leaf extract of A. glabra are flavonoids, glycosides, saponins, tannins, steroids, acidic compounds, and anthraquinones. The alginate films impregnating A. glabra leaf extract exerted wound healing activity and also enhanced the rate of wound contraction. It is believed that the phytochemicals present in the leaf extract play a key role in the promotion of the healing process. Ata dose of 3.0% (w/v) A. glabra leaf extract, burn wounds recovered well without dermalirritation. Administration of the A. glara contained dressing promotes burn healing, as evidenced by decreased healing time and faster wound contraction. It could be stated that A. glabra leaves possess wound healing properties 34.
Larvicidal Efficacy: In recent mosquito control programs, larvicides have been the main tool. The most widely used larvicides organophosphates such as Methoprene, Temephos, and biological control by Bacillusthuringiensis israelensis (Bti). Since the larvicides are applied to either natural or artificial bodies of water, they must be harmless to beneficial and other nontarget organisms, including humans. It is reported that the genus Annona shows strong insecticidal properties. According to reports,A. crassiflora shows larvicidal activity against Ae. Aegypti. A. squamosa shows larvicidal activity against Ae. Aegypti and Culex quinquefasciatus. A. muricata seed extract shows larvicidal activity against Ae. aegypti.
Ethanol stem bark extract of A. glabra is larvicidal to Ae. aegypti. Aqueous leaf extract ofA. glabrashows larvicidal properties on Ae. aegypti and Ae. Albopictus (5.94 mg/L, and 5.00 mg/L respectively). Nanoformulations ofA. glabra silver has shown enhanced larvicidal efficacy on Ae. aegypti and Ae. Albopictus (LC50 = 2.51 mg/L and 2.43 mg/L, respectively) 13.
Wine Production: Pond apple (Annona glabra L.) trees were highly distributed in swamp regions of the Mekong Delta, Vietnam. When ripening, A. glabra fruits turned from green to yellow. Ripen A. glabra fruits contained large phenolic constituents with valuable phytochemical benefits. However, ripened pond apple fruits are not as successfully utilized as other commercial fruits. The quality of wine production from pond apple is affected by the different technical variables of fermentation. Ripen A. glabra fruits were peeled, blended, deseeded, crushed, enzyme-treated (pectinase 25 mg/l), added with sugar (5-13%w/w), pasteurized (sulfite 30 mg/l), inoculated with the yeast Saccharomyces pastoria-nus ratio (0.1-0.5%), and macerated at the temperature 14-22°C in different time (6- 14 days). Malolactic fermentation was carried out in anaerobic condition at 12 °C for different durations (4-20weeks). After the completion of the malolactic fermentation, the wine was racked and clarified with different fining agents like wheat gluten, bentonite, kaolin, polyvinylpyrrolidone, gelatine, and kaolin at 0.03% (v/v). Results showed that must be added with 9% sugar and 0.4% yeast inoculation, fermentation temperature of 16OC in 10 days. Malolactic fermentation could be completed in 12 weeks. During the pond apple wine production, gelatine was shown to be the best candidate among different clarifying agents to remove turbidity in the wine while retaining the most total phenolic content and antioxidant capacity. Fermentation gave a high ethanol content (4.26±0.02%v/v) under the above technical variable conditions. The total phenolic content (32.79±0.00 mg GAE/100 ml), 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging (11.84±0.01 %), overall acceptance (8.34±0.01 score), and low turbidity (24.41±0.00 NTU) were also noticed.
The elevated ethanol level and preservation of phytochemicals were key factors in achieving the favourable sensory rating of pond apple wine, meeting the standards expected for an alcoholic beverage. Ripen Pond apple fruit would be a promising carbohydrate source to convert into a new fruit wine with a pleasant alcoholic flavour and attractive appearance 35.
Anti-oxidant Activity: Antioxidants are compounds which retard or prevent oxidation and prolong the life of oxidizable matter. Free radicals / oxidants play a crucial role in various biochemical processes and are integral to aerobic life and metabolic functions. They have high reactivity, a very short half-life, and damaging activity towards macromolecules like proteins, lipids, and DNA. These species can originate from oxygen or nitrogen 36. A. glabra extract showed moderate antioxidant activity. The standard used was quercetin, a compound that belongs to the most common class of flavonoids. And stands out for its great anti-class of the most common flavonoids and stands out for its great antioxidant potential. The extracts with the best antioxidant activity were ethyl acetate and methanol from the inner bark and methanol from the seeds, which correspond to those containing phenolic compounds 37.
CONCLUSION: Plants are the natural sources of bioactive compounds used to treat various life-threatening diseases. A. glabra showed various phytochemicals, which means that it can be used for treating various diseases. The review shows the activity of various parts of the plant and its pharmacogenetic profile. Extract and phyto-constituents isolated from this plant have been shown to produce different pharmacological responses, which include anti-leishmanial activity, anti-microbial, anti-oxidant, anticancer, anti-inflammatory, wood protection against termites, larvicidal efficacy, burn healing properties, and wine production. Considering all the above medicinal importance of A. glabra, it can be concluded that further studies on this plant may be helpful for further researchers to develop some new drugs.
ACKNOWLEDGMENT: I would like to express my sincere gratitude to Bharathi Education Trust, Bharathinagar, Mandya, Karnataka. For their invaluable support. I am also thankful to Dr. T. Tamizh Mani, Pavithra T and Shiju L, for their full support.
CONFLICTS OF INTEREST: No conflicts of interest.
REFERNCES:
- Sheba PT, Devasia JV and Joseph E: Phytochemical screening and chromatographic identification of acetogenin in Annona glabra L. leaves. Int J Curr Res Chem Pharm Sci 2022; 9(7): 1-7.
- Qazi Majaz A and Molvi Khurshid I: Herbal medicine: A comprehensive Review. Int J pharm Res 2016; 8(2): 1-5.
- Kusmardiyani S, Suharli YA, Insanu M and Fidrianny I: Phytochemistry and pharmacologicalactivities of Annona genus: A review. Curr Res on Bioscences and Biotechnology 2020; 2(1): 77-88.
- Avinash KR, Jyothi MJ and Ashok Kumar CK: Lannea coromendelica The Researchers Tree. J Pharm Res 2011; 4(3): 577-79.
- Biba VS, Amily A, Sangeetha S and Remani P: Anticancer, antioxidant and antimicrobial activity of Annonaceae family. World J Pharm Pharm Sci 2014; 3(3): 1595-604.
- Oliveira LE, Sánchez CM, Monteiro RB and de Medeiros FA: Application of Annona glabra L. (Annonaceae) in the area of health, chemical composition and biological activity. Revista Amazonia Investiga 2022; 11(49): 224-38.
- https://en.wikipedia.org/wiki/Annona_glabra
- https://florida.plantatlas.usf.edu/plant.aspx?id=2172
- http://www.stuartxchange.org/PondApple
- https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.5811
- Annona glabra (lucidcentral.org)
- Stephen H. Brown, Horticulture AgentSusan Mark, AARP Administrative Assistant Lee County Extension, Fort Myers, Florida (239) 533-751.
- Thang TD, Dai DN, Hoi TM and Ogunwande I: A. Study on the volatile oil contents of Annonaglabra L, Annona squamosa L, Annona muricate L, and Annona reticulata L, from Vietnam. J Nat Prod Res 2013; 27(13): 1232–36.
- https://tropical.theferns.info/viewtropical.php?id=Annona%20glabra
- Chang FR, Yang PY, Lin JY, Lee KH and Wu YC: Bioactive kaurane diterpenoids from Annona glabra. J Nat Prod 1998; 61(4): 437-39.
- Chen CY, Chang FR, Cho CP and Wu YC: ent-kaurane diterpenoids from Annona glabra. J Nat Prod 2000; 63(7): 1000-1003.
- Wu YC, Hung YC, Chang FR, Cosentino M, Wang HK and Lee KH: Identification of ent-16β, 17-dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J Nat Prod 1996; 59(6): 635-37.
- Chen Cy, Wu TY, Chang FR and Wu YC: Lignans and kauranes from the stems of Annona cherimola. J Chin Chem Soc 1998; 45(5): 629-34.
- Satake T, Murakami T, Saiki Y and CHEN C: Chemical and chemotaxonomical studies on Filices. LI. Chemical studies on the constituents of costa rican ferns. Chemical and Pharmaceutical Bulletin 1984; 32(11): 4620-24.
- Jossang A, Dubaele B, Cavé A, Bartoli MH, Bériel H:Annomontacine une nouvelle acetogenine γ-lactone-monotetrahydrofurannique cytotoxique de l'Annona montana. J Nat Prod 1991; 54(4): 967-71.
- Gallardo T, Aragon R, Tormo J, Blazquez MA, Zafra-Polo MC and Cortes D: Acetogenins from Annona glabra Phytochemistry 1998; 47(5): 811-16.
- Chan FR, Wu YC, Duh CY and Wang SK: Studies on the acetogenins of Formosan Annonaceous plants, II. Cytotoxic acetogenins fromannona reticulata. J Nat Prod 1993; 56(10): 1688-94.
- Chen CY, Chang FR, Chiu HF, Wu MJ and Wu YC: Aromin-A, an Annonaceous acetogenin from Annona cherimola. Phytochemistry 1999; 51(3): 429-33.
- Chen CY, Chang FR and Wu YC: The constituents from the stems of cherimola. JCCS 1997; 44(3): 313-19.
- Khalaf OM, Osman AF, Abdel-Aziz MS, Elhagrasi AM and Ahmed Ghareeb M: Annona glabra fruit extracts: Chemical profiling and their potential antimicrobial activity against pathogenic microbial strains. Egypt J Chem 2023; 66(2): 495-505.
- Sun L, Zhu JX, Yu JG, Yu DL, Li DY and Zhou LD: Chemical constituents from the seeds of Annona glabra (Annonaceae). Acta Pharma Sinica 2003; 38(1): 32-6.
- Araujo DL, de Sousa Júnior CP, Silva MC, Silva VS, de Abreu Gonçalves S, Lima GM, Evangelista TH, Evangelista HH, de Sousa EO, Neto AA and de Sousa Santos AB: Evaluation of the anti-Leishmania mechanisms of Annona glabra L. (Annonaceae): A brief review. Res Soc Dev2021; 10(8): 2-6.
- Dev AA and Joseph SM: Anticancer potential of Annona genus: A detailed review. J Indian Chem Soc 2021; 98(12): 100-231.
- Zhang YH, Peng HY, Xia GH, Wang MY and Han Y: Anticancer effect of two diterpenoid compounds isolated from Annona glabra Linn. Acta Pharmacologica Sinica 2004; 25(7): 937-42.
- Sophiya P, Lohith NS, Giresha AS, Shankar J, Meti RS and Dharmappa KK: Inhibition of GIIA sPLA2 by Annona glabra as an anti-inflammatory function: An attempt to neutralize the inflammation of cancer. Biomedicine 2020; 40(1): 45-50.
- Sivangnanam DS, Ram M and Ram Krishna Rao M: In-vitro inhibitory effects of Annona glabra on selected human pathogens. J Chem Pharm Res 2016; 8(1): 1-6.
- Siebra CA, Nardin JM, Florão A, Rocha FH, Bastos DZ, Oliveira BH and Weffort-Santos AM: Anti-inflammatory potential of Annona glabra, Annonaceae. Revista Brasileira de Farmacognosia 2009; 19(1A & 1B): 82-88.
- Priadi T, Chotimah N and Ismanto A: Bioactivity Analysis of Annona glabra Seed Ectracts for Wood Protection against Termites (Cryptotermes Cynocephalus Light. And Coptotermes Curvignathus Holmgren.). Indones J for Res 2021; 8(2): 127-34.
- Le Son H and Nguyen TT: Preparation and evaluation of Annona glabra L. leaf extract contained alginate film for burn healing. European Journal of Medicinal Plants 2013; 3(4): 485-99.
- Minh NP: Wine production from ripen pond apple (Annona glabra L.) fruit. Plant Science Today 2022; 9(2): 454-60.
- Panchawat S, Rathore KS and Sisodia SS: A review on herbal antioxidants. IJPTR 2010; 2(1): 232-39.
- Silva AA, Alexandre JB, Vieira LG, Rodrigues SP, Falcão MJ and de Morais SM: Estudo fitoquimico e atividades leishmanicida, anticolinestarásica e antioxidante de extratos de Annona glabra L. (araticum panã). Revista de Ciências Farmacêuticas Básica e Aplicada 2015; 36(2): 189-94.
How to cite this article:
Sinchana R, Mani TT, Pavithra T and Shiju L: A review on a miracle plant Annona glabra Linn. Int J Pharmacognosy 2024; 11(3): 65-77. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.11(3).65-77.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
65-77
1282 KB
1315
English
IJP
R. Sinchana *, T. Tamizh Mani, T. Pavithra and L. Shiju
Department of Pharmacognosy, Bharathi College of Pharmacy, Bharathinagara, Mandya, Karnataka, India.
sinchanagowda802@gmail.com
14 February 2024
19 March 2024
27 March 2024
10.13040/IJPSR.0975-8232.IJP.11(3).65-77
31 March 2024