A NEW NEO-CLERODANE DITERPENOID FROM TEUCRIUM POLIUM LINN.
HTML Full TextA NEW NEO-CLERODANE DITERPENOID FROM TEUCRIUM POLIUM LINN.
M. W. M. Sadaka
Department of Chemistry, Applied Science Faculty, University of Kalmoon, Deir Atteia, Syria.
ABSTRACT: Teucrium polium L. is a member of the Lamiaceae family and is represented in the Flora of Syria by two variety: Teucrium polium var. angusti folium and Teucrium polium var. mollissimum. Teucrium polium is a wild-growing flowering plant, found abundantly in Syria, and it is used traditional medicine for it's diuretic, antipyretic, antispasmodic, tonic, anti-inflammatory, antihypertensive, anorexic, analgesic, antibacterial and antidiabetic effects. Several reports have demonstrated a wide range of beneficial biological and pharmacological activities of the phenylethanoid, phenylpropanoid, and flavonoid components, while the furano neo-clerodane diterpenoids present in germander have been implicated in the in-vivo hepatotoxicity of this botanical. Phytochemical studies of this plant have been carried out. Aerial parts of the plant were extracted with petroleum ether at room temperature, and then extract with methanol in Soxhlet, and finally extract with aqueous methanol at room temperature successively. Fractionation of the methanol extract by column chromatography and purification by crystallization yielded three known flavonoids Cirsimartin 2, Cirsiliol 3, and Apigenin 4. The aqueous methanolic extract yielded one new neo-clerodane type diterpenoid, Syrapolin I 1 (3,12- diacetoxy - 4α,18α ; 15,16- diepoxy - 6 – oxo – neo – cleroda - 13 (16), 14- diene -7α, 20β- dihydroxy-19- hemiacetal). The structure of compounds was proposed based on spectroscopic methods IR, MS, and 1-D (1H, 13C, and DEPT) and 2-D (COSY, HETCOR, HMBC) NMR experiment, and comparison with closely related compounds.
| Keywords: |
Teucrium polium L., neo-clerodane, Diterpenoid, Cirsimartin, Cirsiliol, Apigenin
INTRODUCTION: Teucrium polium (Family Lamiaceae) is one of the popular fragrant plants in Syria and is distributed throughout the country. In the Flora of Syria, this genus is represented by 21 species such as T. creticum grow in Safita and Mussiaf, T. oliverianum grows in Deir-ez-Zor, T. chamaedrys grow in Lattakia and Banias, and T. polium grow in Al-Hassaka. Teucrium polium is a perennial shrub, 20-50 cm high, distributed widely in the dry and stony hills and deserts of almost all Mediterranean countries, South-western Asia, Europe, and North Africa 1.
The bruised foliage releases a pleasant aromatic odor and the flowers are small, in clusters and range from pink to white. In the folk medicinal traditions, this plant is used for its antibacterial, anti-spasmodical, anti-rheumatic, anti-diabetic and antipyretic activities. In an admixture with other powdered herbs, it is claimed to be therapeutic for peptic ulcer.
The biological activity of T. polium is widely reported, and it has been shown to possess anti-inflammatory 2, anti-nociceptive 3, 4 activities. The aqueous extract of Teucrium polium aerial parts, given intraperitoneally at dosages from 50 to 150 mg/kg for 10 days, reduced significantly the serum levels of cholesterol and triglycerides in hyperlipidemic rats 5. Recently, the high insulinotropic and anti-hyperglycemic activity of its crude extract using both animal and isolated rat pancreatic islets has been evaluated 6-8.
Antibacterial activity against some Gram-positive and Gram-negative bacteria as well as antifungal activity against Yarrovia lipolitica and Saccharomyces cerevisiae has also been reported 9, 10. Recently, cytotoxic, anticancer, and antimutagenic effects of ethanol and aqueous extracts of T. polium have been shown on various cell lines 11-13. T. polium extract contains selenium 14 and has shown antioxidant activity like α-tocopherol. In particular, their antioxidant activities were evaluated through the use of several commonly accepted in vitro assays, which included DPPH scavenging (DPPH), reducing power (RP), xanthine oxidase inhibitor effect (XOI) and antioxidant activity in a linoleic acid system (ALP) 15-21.
The few adverse effects of T. polium have been reported which indicate the relatively safe nature of this medicinal herb 22-27. The composition of the essential oil of T. polium has been the subject of several investigations 28-36. The naturally occurring neo-clerodane diterpenoids have attracted interest because of their biological activity as insect antifeedant and as antifungal, anti-tumor, and antimicrobial agents. Although a large number of these compounds have been isolated from many plants in the last few years, the genus Teucrium is one of the richest sources of neo-clerodane diterpenes: more than 230 diterpenes have been described. Phytochemical investigations of T. polium have been shown that it contains a lot of diterpenes belong to neo-clerodane type 37-45.
T. polium also rich in constituents such as flavonoids, iridoids and Phenylpropanoid glycosides with undoubted antioxidant properties 18, 46-50. Our previous work on the aerial parts of T. polium L. var. mollissimum Hand-Mazz. led to the isolation of one new neo-clerodane diterpenes Syrapolin II, and two known compounds 6'-O-caffeoyl-8-O-acetylharpagide, clerosterol-3-O-glucoside 51. In continuation of our studies on neo-clerodane diterpenoids from the Teucrium species, we have investigated T. podium, a small shrub which grows widely in Syria. From the methanolic extract and aqueous methanolic extract of the aerial parts of this plant we have isolated the new neo-clerodane diterpenoids Syraoiln I 1, beside to three new known flavonoids, Cirsimartin 2, Cirsiliol 3, and Apigenin 4.
MATERIALS AND METHODS:
Plant Material: Teucrium polium L. Amouda, Al-Hassaka, Syria in April 2008, and identified by Pro. Dr. Anwer Al-Khateeb, Department of Botany, College of Science, Damascus University, Damascus, Syria. A voucher specimen is available in the herbarium of Department of Botany in Damascus University.
Chemicals: Silica gel 60 F254 plates (Merck) were used for analytical and preparative TLC. Silica gel S (Merck; Mesh 230-400) was used for column chromatography (CC), and all the chemicals were purchased from Merck.
Instrumentation: The 1H and 13C NMR spectra were recorded on Bruker Ultra Shield 400 MHz spectrometer (Faculty of Science, Al-Baath University, Homs, Syria) and reported in ppm. (δ) relative to TMS as internal standard and DMSO-d6 as a solvent. The IR spectra were measured on JASCO (FT-IR)-410 spectrophotometer (Medico Medical Company, Homs, Syria). MS spectra were recorded on Shimadzu GCMS-QP 2010 (Syrian atomic energy commission, Damascus, Syria).
Experimental:
Extraction and Isolation: Dried and finely powdered Teucrium polium L. aerial parts (4 kg) were extracted with petroleum ether at room temperature for 2 days, then filtrate and the residual plant re-extracted with methanol in soxhlet. The methanolic extract (180 g) evaporated under reduced pressure and then extracted with diethyl ether. The diethyl ether extract (8g) was chromatographed on silica gel column and eluted with n-hexane, n-hexan-CHCl3 (90:10), n-hexan-CHCl3 (50:50), gradient CHCl3-MeOH, and pure MeOH, and the fractions collected as 25 ml. Elution with CHCl3-MeOH (90:10) afford 160 fractions. Fractions (92-100) gave 71 mg of an impure compound which were crystallized in dichloromethane to give 38 mg of compound 2, and Fractions (122-124) gave 64 mg of an impure compound which were crystallized in dichloromethane to gave 29 mg of compound 3. Elution with CHCl3-MeOH (80:20) gave 48 mg residues which were crystallized in a mixture of acetone-methanol to gave 22 mg of pure compound 4. The plant residue from methanolic extract then re-extracted with the mixture of MeOH-H2O (70:30) at room temperature, and the filtrate was evaporated in vacuum, and during this evaporation, the white precipitate was accumulated, we filtrate it and washed with methanol to gave 80 mg of pure compound 1.
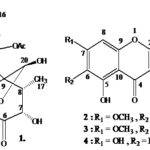
RESULTS AND DISCUSSION: Syrapolin I (1) (Fig. 1) gave a molecular ion peak in its EI-mass spectrum at m/z 478, in agreement with the molecular formula C24H30O10. The other prominent peaks were found to occur at m/z 460 [M-H2O]+, 418 [M-AcOH]+, 135, 122, 105(100), 94, 91, 81, 77, 55, 43. The IR spectrum of (1) showed absorptions of furan ring (3141.5, 3124.1, 1504.2, 874.6 cm-1), hydroxyl (3381.5 cm-1, br), tow acetates (1754.9, 1731.8, 1280.5, 1239.0 cm-1), and a strong band for a carbonyl group (1713.4 cm-1).
FIG. 1: STRUCTURE OF COMPOUNDS 1, 2, 3, 4
The data from the 13C-NMR spectrum Table 1 display 24 signals. The multiplicities of carbon signals were determined by the DEPT experiment. Taking into account the results from our comprehensive 1D and 2D-NMR studies and previous knowledge derived from metabolites isolated from the genus Teucrium 39, 51-53. It is evident that (1) possesses a highly oxidized neo-clerodane nucleus. The 1H-NMR spectrum (Table 1) of compound (1) showed add at δ 6.56 (J = 0.8, 1.6 Hz, H-14), a broad singlet at δ 7.71 (H-15), and a triplet at δ 7.63 (J = 1.7 Hz, H-16) were readily assigned to a terminal furan ring.
Also, the 1H-NMR spectrum showed two AB system (δ 2.95, 4.14 and 2.96, 2.81) which we have assigned to H-19 and H-18 respectively. The broad doublet at δ 5.83 and the doublet at 4.87 were assigned to H-12 and H-20, respectively. The 1H-NMR spectrum also showed two doublets at δ 5.03 (5.6 Hz), 6.47 (4.2 Hz) which were not connected to any carbon atoms in HETCOR spectrum were assigned to two hydroxyl group, and disappeared from 1H-NMR spectrum when recorded in DMSO with few drops of D2O. Detailed examination of 1H-NMR spectrum indicated the presence of 1H-1H spin system which could be traced from the secondary methyl protons δ 1.09 (d, J = 6.8 Hz, 3H-17) to δ 1.66 (q, J = 6.4 Hz, H-8) and from there to an oxymethine proton at δ 4.25 (dd, J = 5.6, 9.9 Hz, H-7). Mutual coupling with the signal at δ 5.03 (d, J = 5.6 Hz, OH) suggested by the common J value, was confirmed by a cross peak in the 1H-NMR, 2D COSY spectrum, and supported the presence of a hydroxyl group at C-7 with α configuration (equatorial). As expected, no cross peak was observed in the HETCOR spectrum for a carbon resonance and proton signal at δ 5.03, but in the HMBC spectrum, cross peaks were observed for C-6, C-8, and C-7. The HMBC spectrum also showed cross peaks between the signal at δ 6.47 (d, J = 4.2 Hz, C20-OH) and C-20, C-9, and C-10. The 1H-NMR spectrum also shows tow singlet at δ 1.90, and 2.03 indicate to the tow CH3 in acetate groups.
The 13C-NMR Table 1 and DEPT spectra confirmed the presence of a furan ring (δ 126.70,
C-13; 109.38, C-14; 140.37, C-15 and 144.31, C-16), a carbonyl group (δ 208.23, C-6), tow acetate (δ 169.90 COMe; 21.42, COMe, and 170.24 COMe; 21.70, COMe), one methyl signal (δ 13.68, C-17) and two oxygen-substituted methylene signals for the C-18 and C-19 at δ 52.39 and 54.00 respectively, three oxygen-substituted methine signals for the C-3, C-7, C-12 and C-20 at δ 72.97, 78.85, 65.23 and 93.29 respectively. On the basis of these facts, the structure of (1) was established as 3, 12-diacetoxy-4α, 18α; 15, 16-diepoxy-6-oxo-neo-cleroda-13(16),14-diene-7α,20β-dihydroxy-19-hemiacetal.
TABLE 1: 13C-NMR AND 1H-NMR DATA OF 1 (400 MHz, DMSO-D6)
| S. no. | C | DEPT | HETCOR |
| 1 | 19.05 | CH2 | 2.17m (5.2 Hz) |
| 2 | 26.87 | CH2 | a 1.62 brd (6.4 Hz)
b 2.14m (5.2 Hz) |
| 3 | 72.97 | CH | 4.29d (6.1 Hz) |
| 4 | 55.93 | C | |
| 5 | 48.20 | C | |
| 6 | 208.23 | C | |
| 7 | 78.85 | CH | 4.25dd (5.6, 9.9 Hz) |
| 8 | 46.90 | CH | 1.66q (6.4 Hz) |
| 9 | 41.12 | C | |
| 10 | 40.77 | CH | 2.20d (4.4 Hz) |
| 11 | 35.73 | CH2 | a 1.56dd (16.2, 2.4 Hz)
b 2.45dd (12, 16 Hz) |
| 12 | 65.23 | CH | 5.83brd (8 Hz) |
| 13 | 126.70 | C | |
| 14 | 109.38 | CH | 6.56dd (0.8, 1.6 Hz) |
| 15 | 140.37 | CH | 7.71brs |
| 16 | 144.31 | CH | 7.63t (1.7 Hz) |
| 17 | 13.68 | CH3 | 1.09d (6.8 Hz) |
| 18 | 52.39 | CH2 | a 2.81d (4.7 Hz)
b 2.96d (4.7 Hz) |
| 19 | 54.00 | CH2 | a 2.95d (12.3 Hz)
b 4.14d (12.4 Hz) |
| 20 | 93.29 | CH | 4.87d (4.3 Hz) |
| CH3CO | 21.42 | CH3 | 1.88 s |
| CH3CO | 21.70 | CH3 | 2.04 s |
| CH3CO | 169.90 | ||
| CH3CO | 170.24 | ||
| OH | 5.03d (5.6 Hz) | ||
| OH | 6.47d (4.2 Hz) |
Compound (2) was obtained as a yellow granular powder and exhibiting a positive ferric chloride test and magnesium hydrochloric acid test. Compound (2) gave a molecular ion peak in its EI-mass spectrum at m/z; M = 314 (100%), and M+1 (11%) in agreement with the molecular formula C17H14O6. IR spectrum of (2) showed absorption band for hydroxyl groups 3300 cm-1, α, β-unsaturated carbonyl carbon 1640 cm-1, and aromatic double bond 1595 cm-1 functionalities. The UV spectrum of (2) exhibited absorption maxima at 331 (Band I) and 275 nm (Band II) (in MeOH), bathochromic shifts of 67, 3 and 4 nm with NaOMe, NaOAc and AlCl3 in Band I, respectively. These data revealed that (2) has a flavonoid type structure.
The 1H-NMR spectrum of (2) Table 2 showed a characteristic low-frequency signal at δ 12.90 (s, 5-OH) assignable to aromatic hydroxyl signal hydrogen bonded to carbonyl at C-5 of a flavonoid. Two ortho-coupled aromatic protons at δ 7.92 (1H, d, J = 8.1 Hz) and δ 6.92 (1H, d, J = 8.1 Hz) were diagnostic for a C-2'/C-6' and C-3'/C-5' oxygenated ring B. The 1H NMR spectrum showed two aromatic proton signals at (δ 6.86, s, H-8) and (δ 6.80, s, H-3). The 1H-NMR also showed two singlet signal at δ 3.72 (s, 3H) and δ 3.90 (s, 3H) indicate the presence of two methoxy group. In 13C-NMR spectra Table 2 and DEPT-135, the signals at δ 164.53, 103.15, 182.10, 152.58 and 105.56 were typical of C-2, C-3, C-4, C-9 and C-10 of a flavonoid moiety. The position of two methoxy groups was authenticated through the HMBC spectrum between methoxy protons at δ 3.72 and C-6, and δ 3.90 and C-7. By comparison of 1H- and 13C-NMR chemical shifts with those reported in the literature, the flavonoid skeleton were in agreement to those of cirsimartin 54.
TABLE 2: 13C-NMR AND 1H-NMR DATA OF COMPOUND (2), (400 MHZ, DMSO-D6)
| No | 2 | 3 | 4 | |||
| C | H | C | H | C | H | |
| 2 | 164.53 | --- | 164.77 | --- | 164.77 | --- |
| 3 | 103.15 | 6.80, 1H, s | 103.18 | 6.71, 1H, s | 103.32 | 6.76, 1H, s |
| 4 | 182.10 | --- | 183.64 | --- | 182.25 | --- |
| 5 | 153.11 | --- | 153.13 | --- | 164.23 | --- |
| 6 | 132.33 | --- | 132.35 | --- | 99.44 | 6.18, 1H, d,
J = 2.09 Hz |
| 7 | 159.09 | --- | 159.11 | --- | 164.95 | --- |
| 8 | 92.00 | 6.86, 1H, s | 91.95 | 6.85, 1H, s | 94.54 | 6.48, 1H, d,
J = 2.09 Hz |
| 9 | 152.58 | --- | 152.57 | --- | 157.86 | --- |
| 10 | 105.56 | --- | 105.54 | --- | 104.14 | --- |
| 1' | 121.61 | --- | 121.94 | --- | 121.68 | --- |
| 2' | 129.01 | 7.92, 1H, d,
J = 8.1 Hz |
113.94 | 7.45, 1H, s | 128.99 | 7.92, 1H, d,
J = 8.9 Hz |
| 3' | 116.47 | 6.92, 1H, d,
J = 8.1 Hz |
146.30 | --- | 116.50 | 6.92, 1H, d,
J = 8.9 Hz |
| 4' | 161.79 | --- | 150.38 | --- | 161.99 | --- |
| 5' | 116.47 | 6.92, 1H, d,
J = 8.1 Hz |
116.49 | 6.90, 1H, d,
J = 8.1 Hz |
116.50 | 6.92, 1H, d,
J = 8.9 Hz |
| 6' | 129.01 | 7.92, 1H, d,
J = 8.1 Hz |
119.59 | 7.44, 1H, d,
J = 8.1 Hz |
128.99 | 7.92, 1H, d,
J = 8.9 Hz |
| OCH3 | 60.53 | 3.72, 3H, s | 60.58 | 3.72, 3H, s | 12.98, s | |
| OCH3 | 56.91 | 3.90, 3H, s | 56.93 | 3.91, 3H, s | ||
| C4'-OH | 10.40, br s | 12.91, s | ||||
| C5-OH | 12.90, s | |||||
Compound (3) Fig. 1 isolated as a pale yellow amorphous powder. The UV spectrum of (3) in methanol showed three peaks at 274, 348 and 447 nm. The UV spectrum of (3) with NaOMe showed peaks at 266 and 408 nm, and with NaOAc at 254 and 338 nm, and with AlCl3 at 271 nm. These data revealed that (3) has a flavonoid type structure. The IR spectrum of (3) showed absorption band for hydroxyl groups 3410 cm-1, carbonyl group 1647 cm-1 (C=O α,β-unsaturated), and an aromatic double bond at 1580 cm-1. Compound (3) gave a molecular ion peak in its EI-mass spectrum at m/z 330 (100%), in agreement with the molecular formula C17H14O7.
The 1H-NMR spectrum of (3) Table 2 showed a characteristic low-frequency signal at δ 12.91 (s, OH) assignable to aromatic hydroxyl. Two ortho-coupled aromatic protons at δ 7.44 (1H, d, J = 8.1 Hz) and δ 6.90 (1H, d, J = 8.1 Hz) were diagnostic for a C-5' and C-6' oxygenated ring B. The 1H-NMR spectrum showed three aromatic proton signals at (δ 6.85, s, H-8), (δ 6.71, s, H-3) and (δ 7.45, s, H-2'). The 1H-NMR also showed two singlet signal at δ 3.72 (s, 3H) and δ 3.91 (s, 3H) indicate the presence of two methoxy group. In 13C-NMR spectrum Table 2 and DEPT-135, the signals at δ 164.77, 103.18, 183.64, 152.58 and 105.54 were typical of C-2, C-3, C-4, C-9 and C-10 of a flavonoid moiety. The position of two methoxy group was authenticated through the HMBC spectrum between methoxy protons at δ 3.72 and C-6, and δ 3.91 and C-7. By comparison of 1H- and 13C-NMR chemical shifts with those reported in the literature, the flavonoid skeleton were in agreement with those of Cirsiliol 55.
Compound (4) Fig. 1 isolated as a yellow granular powder. The UV spectrum of (4) in methanol showed three peaks at 266 and 334 nm. The UV spectrum of (4) with NaOMe showed peaks at 274, 322 and 390 nm, and with NaOAc at 276, 300 and 378 nm, and with AlCl3 at 277, 302, 338 and 384 nm. These data revealed that (4) has a flavonoid type structure. The IR spectrum of (4) showed absorption band for hydroxyl groups 3352 cm-1, α, a β-unsaturated carbonyl group 1647 cm-1, and an aromatic double bond at 1602 cm-1. Compound (4) gave a molecular ion peak in its EI-mass spectrum at m/z 270 (100%), in agreement with the molecular formula C15H10O5.
The 1H-NMR spectrum of (4) Table 2 showed two ortho-coupled aromatic protons at δ 7.92 (1H, d, J = 8.9 Hz) and δ 6.92 (1H, d, J = 8.9 Hz) were diagnostic for a C-2'/C-6' and C-3'/C-5' oxygenated ring B. The 1H-NMR spectrum of (4) Table 2 also showed two metha-coupled aromatic protons at δ 6.48 (1H, d, J = 2.09 Hz) and δ 6.18 (1H, d, J = 2.09 Hz) were diagnostic for a C-8 and C-6 oxygenated ring A. The 1H-NMR also showed one singlet signal at δ 6.76 (s, 1H) for C-3. In 13C-NMR spectrum Table 2 and DEPT-135, the signals at δ 164.77, 103.32, 182.25, 157.86 and 104.14 were typical of C-2, C-3, C-4, C-9 and C-10 of a flavonoid moiety. Analysis of the 1H-NMR, 13C-NMR, COSY, HETCOR, and HMBC experiments indicated that this compound bears a flavonoid skeleton, which was identified as Apigenin, by comparison of 1H- and 13C-NMR chemical shifts with those reported in the literature 56.
CONCLUSION: Phytochemical study of the aerial parts of Teucrium polium resulted in the isolation of four compounds, a new neo-clerodane diterpenoid (1), and three known flavonoids. The known flavonoids were identified as Cirsimartin (2), Cirsiliol (3), and Apigenin (4). The structures were determined by physical, chemical and spectral techniques.
ACKNOWLEDGEMENT: The author is very grateful to Prof. Dr. Anwer Al-khateeb (Botany Department, Faculty of Science, Damascus University) for plant taxonomic identification. The author also grateful to the mass spectra unit at the Department of Chemistry, Atomic Energy Commission of Syria.
CONFLICT OF INTEREST: We declare that we have no conflict of interest.
REFERENCES:
- Mouterde P: Nouvelle Flore du Liban et de la Syrie. Dar el-Machreq, Beirut 1983; 3.
- Menichini F, Conforti F, Rigano D, Formisano C, Piozzi F and Senatore F: Phytochemical composition, anti-inflammatory and antitumor activities of four Teucrium essential oils from Greece. Food Chemistry 2009; 115: 679-686.
- Abdollahi M, Karimpour H and Monsef-Esfehani HR: Antinociceptive effects of Teucrium polium total extract and essential oil in mouse writhing test. Pharmacol Res 2003; 48: 31-35.
- Baluchnejadmojarad T, Roghani M and Roghani-Dehkordi F: Antinociceptive effects of Teucrium polium leaf extract in the diabetic rat formalin test. J Ethnopharmacol 2005; 97: 207-210.
- Rasekh HR, Khoshnood-Mansourkhani MJ and Kamalinejad M: Hypolipidemic effects of Teucrium polium in rats. Fitoterapia 2001; 72: 937-939.
- Afifi FU, Al-Khalidi B and Khalil E: Studies on the in-vivo hypoglycemic activities of two medicinal plants used in the treatment of diabetes in Jordanian traditional medicine following intranasal administration. J Ethnopharmacol 2005; 14: 314-318.
- Esmaeili MA and Yazdanparast R: Hypoglycaemic effect of Teucrium polium: studies with rat pancreatic islets. J Ethnopharmacol 2004; 95: 27-30.
- Vessal M, Zal F and Vasei M: Effects of Teucrium polium on oral glucose tolerance test, regeneration of pancreatic islets and activity of hepatic glucokinase in diabetic rats. Arch Iranian Med 2003; 6: 35-39.
- Autore G, Capasso F, De Fusco R, Fasulo MP, Lembo M, Mascolo N and Menghini A: Antipyretic and antibacterial actions of Teucrium polium Pharmacol Res Commun 1984; 16: 21-29.
- Aggelis G, Athanassopoulos N, Paliogianni A and Komaitis M: Effect of a Teucrium polium extract on the growth and fatty acid composition of Saccharomyces cerevisiae and Yarrowia lipolytica. Antonie Van Leeuwenhoek 1998; 73: 195-198.
- Khader M, Eckl PM and Bresgen N: Effects of aqueous extracts of medicinal plants on MNNG-treated rat hepatocytes in primary cultures, J Ethnopharmacol 2007; 112: 199-202.
- Khader M, Bresgen N and Eckl PM: Antimutagenic effects of ethanolic extracts from selected Palestinian medicinal plants. J Ethnopharmacol 2010; 127: 319-324.
- Nematollahi-Mahani SN, Rezazadeh-Kermani M, Mehrabani M and Nakhaee N: Cytotoxic effects of Teucrium polium on some established cell lines. Pharm Biol 2007; 45: 295-298.
- Jurisic RV, Kalodera Z and Grgic J: Determination of selenium in Teucrium generation atomic absorption spectrometry. Z Naturforsch 2003; 58: 143-145.
- De Marino S, Festa C, Zollo F, Incollingo F, Raimo G, Evangelista G and Iorizzi M: Antioxidant activity of phenolic and phenylethanoid glycosides from Teucrium polium. Food Chemistry 2012; 133: 21-28.
- Tepe B, Degerli S, Arslan S, Malatyali E and Sarikurkcu C: Determination of chemical profile, antioxidant, DNA damage protection and antiamoebic activities of Teucrium polium and Stachys iberica. Fitoterapia 2011; 82: 237-246.
- Hasani P, Yasa N, Vosough-Ghanbari S, Mohammadirad A, Dehghan G and Abdollahi M: In-vivo antioxidant potential of Teucrium polium as compared to a-tocopherol. Acta Pharm 2007; 57: 123-129.
- Sharififar F, Dehghn-Nudeh G and Mirtajaldini M: Major flavonoids with antioxidant activity from Teucrium polium Food Chemistry 2009; 112: 885-888.
- Esmaeili MA, Zohari F and Sadeghi H: Antioxidant and protective effects of major flavonoids from Teucrium polium on beta-cell destruction in a model of streptozotocin-induced diabetes. Planta Med 2009; 75: 1418-1420.
- Ardestani A, Yazdanparast R and Jamshidi S: Therapeutic effects of Teucrium polium extract on oxidative stress in the pancreas of streptozotocin-induced diabetic rats. J Med Food 2008; 11: 525-532.
- Suboh SM, Bilto YY and Aburjai TA: Protective effects of selected medicinal plants against protein degradation, lipid peroxidation and deformability loss of oxidatively stressed human erythrocytes. Phytother Res 2004; 18: 280-284.
- Boumerfeg S, Baghiani A, Djarmouni M, Ameni D, Adjadj M, Belkhiri F, Charef N, Khennouf S and Arrar L: Inhibitory Activity on Xanthine Oxidase and Antioxidant Properties of Teucrium polium Extracts. Chinese Medicine 2012; 3: 30-41.
- Starakis I, Siagris D, Leonidou L, Mazokopakisb E, Tsamandasc A and Karatza C: Hepatitis caused by the herbal remedy Teucrium polium Eur J Gastroenterol Hepatol 2006; 18: 681-683.
- Mattei A, Rucay P, Samuel D, Feray C, Reynes M and Bismuth H: Liver transplantation for severe acute liver failure after herbal medicine (Teucrium polium) administration. J Hepatol 1995; 22: 597.
- Larrey D, Vial T, Pauwels A, Castot A and Michel H: Hepatitis after germander (Teucrium chamaedrys) administration, another instance of herbal medicine hepatotoxicity. Ann Intern Med 1992; 117: 129-132.
- Mattei A, Bizollon T and Charles JD: Atteinte hepatique associee a la prise d'un produit de phytotherapie contenant de la germadree petit-chene. Gastroenterol Clin Biol 1992; 16: 798-800.
- Wassel GM and Ahmed SS: On the essential oil of Teucrium polium Pharmazie 1974; 29: 351-352.
- Hassan MM, Muhtadi FJ and Al-Badr AA: GLC-mass spectrometry of Teucrium polium J Pharm Sci 1979; 68: 800-801.
- Cakir A, Duru ME, Harmandar M, Ciriminna R, Passannanti S and Kazlm K: Volatile constituents of Teucrium polium from Turkey. J Essent Oil Res 1998; 10: 113-115.
- Eikani MH, Goodarznia L and Mirza M: Comparison between the essential oil of supercritical carbon dioxide extract of Teucrium polium J Essent Oil Res 1999; 11: 470-472.
- Perez-Alonso MJ, Velasco-Negueruela A and Lopez-Saez JA: The essential oils of two Iberian Teucrium species. J Essent Oil Res 1993; 5: 397-402.
- Aburjai T, Hudaib M and Cavrini V: Composition of the essential oil from Jordanian germander Teucrium polium J Essent Oil Res 2006; 18: 97-99.
- Sadeghia H, Jamalpoorb S and Shirzadib MH: Variability in essential oil of Teucrium polium of different latitudinal populations. Industrial Crops and Products 2014; 54: 130-134.
- Djabou N, Lorenzi V, Guinoiseau E, Andreani S, Giuliani MC, Desjobert JM, Bolla JM, Costa J, Berti L, Luciani A and Muselli A: Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Control 2013; 30: 354-363.
- Purnavaba S, Ketabchia S and Rowshan V: Chemical composition and antibacterial activity of methanolic extract and essential oil of Iranian Teucrium polium against some of phytobacteria. Natural Product Research 2015; 29: 1376-1379.
- Eguren L, Perales A, Fayos J, Savona G, Paternostro M, Piozzi F and Rodriguez B: New Clerodane Diterpenoid from Teucrium polium subsp. aureum X-ray Structure Determination. J Org Chem 1981; 46: 3364-3367.
- Malakov P, Papanov G and Ziesche J: Teupolin III A Furanoid Diterpene from Teucrium polium. Phytochemistry 1982; 21: 2597-2598.
- Malakov P and Papanov G: Furanoid Diterpene from Teucrium polium. Phytochemistry 1983; 22: 2791-2793.
- Fernandez P, Rodriguez B, Savona G and Piozzi F: Neo-Clerodane diterpenoids from Teucrium polium subsp capitatum. Phytochemistry 1986; 25: 181-184.
- De la Torre M, Piozzi F, Rizk AF, Rodriguez B and Savona G: 19-Acetylteupolin IV, A neo-Clerodane diterpenoid from Teucrium polium subsp pilosum. Phytochemistry 1986; 25: 2239-2240.
- Malakov P, Boneva IM, Papanov G and Spassov SL: Teulamifin B, A neo-Clerodane diterpenoid from Teucrium lamiifolium and polium. Phytochemistry 1988; 27: 1141-1443.
- Alcazar R, De La Torre MC, Rodriguez B, Bruno M, Piozzs F and Arnold GNA: Neo-Clerodane Diterpenoids from three Species of Teucrium. Phytochemistry 1992; 31: 3957-3960.
- Bruno M, Maggioa AM, Piozzi F, Puech S, Rosselli SG and Simmonds MSJ: Neoclerodane diterpenoids from Teucrium polium polium and their antifeedant activity. Biochemical Systematics and Ecology 2003; 31: 1051-1056.
- Malakov P, Papanov GG and Mollov NM: Montanin A and B, new furanoid diterpenes of nor-clerodane type from Teucrium montanum Tetrahedron Letters 1978; 19: 2025-2026.
- D’Abrosca B, Pacifico S, Scognamiglio M, D’Angelo G, Galasso S, Monaco P and Fiorentino A: A new acylated flavone glycoside with antioxidant and radical scavenging activities from Teucrium polium Natural Product Research 2013; 27: 356-363.
- Rizk AM, Hammouda FM, Rimpler H and Kamel A: Iridoids and flavonoids of Teucrium polium Planta Medica 1986; 52: 87-88.
- Verykokidou-Vitsaropoulou E and Vajias C: Methylated flavones from polium, Planta Med 1986; 52: 401-402.
- Kawashty SA, Gamal El-din EM and Saleh NAM: The flavonoid chemo-systematics of two Teucrium species from Southern Sinai Egypt. Biochemical Systematics and Ecology 1999; 27: 657-660.
- Oganesyan GB, Galstyan AM, Mnatsakanyan VA, Shashkov AS and Agababyan PV: Phenylpropanoid Glycosides of Teucrium polium. Khimiya Prirodnykh Soedinenii 1977; 1: 630-634.
- Moustapha C, Hasen T and Sadaka MWM: Chemical Constituents of Teucrium polium var. mollissimum Hand-Mazz, Jordan J Chem 2011; 6: 339-345.
- Malakov P, Papanov G and Ziesche J: Teupolin III, A furanoid diterpene from Teucrium polium, Phytochemistry 1982; 21: 2597-2598.
- De la Torre MC, Piozzi F, Rizk AF, Rodriguez B and Savona G: 19-Acetylteupolin IV, A neo-clerodane diterpenoid from Teucrium polium Pilosum. Phytochemistry 1986; 25: 2239-2240.
- Yim SH, Kim HJ and Lee IS: A Polyacetylene and Flavonoids from Cirsium rhinoceros. Arch Pharm Res 2003; 26: 128-131.
- Marder M, Viola H, Wasowaki C, Wolfman C, Waterman PG, Medina JH and Paladini AC: Cirsiliol and caffeic acid ethyl ester, isolated from Salvia guaranitica, are competitive ligands for the central benzodiazepine receptors. Phytomedicine 1996; 3: 29-31.
- Kim NM, Kim J, Chung HY and Choi JS: Isolation of luteolin 7-o-rutinoside and esculetin with potential antioxidant activity from the aerial parts of Artemisia montana. Arch Pharm Res 2000; 23: 237-239.
How to cite this article:
Sadaka MWM: A new neo-clerodane diterpenoid from Teucrium polium L.. Int J Pharmacognosy 2017; 4(6): 185-92. doi link: http://dx. doi.org/10.13040/IJPSR.0975-8232.IJP.4(6).185-92.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.