A COMPREHENSIVE REVIEW ON PRODRUGS OF BIOACTIVE OF NATURAL ORIGIN
HTML Full TextA COMPREHENSIVE REVIEW ON PRODRUGS OF BIOACTIVE OF NATURAL ORIGIN
Ankita Chandwani and Mamta B. Shah *
Department of Pharmacognosy, L. M. College of Pharmacy, Navrangpura, Ahmedabad, Gujarat, India.
ABSTRACT: Prodrugs are bio-reversible, inactive drug by products that can convert into a parent drug inside the body. Around 10% of the medications accepted globally can be categorized as prodrugs. Prodrugs are designed to enhance the site-selective delivery of an active drug by modifying the physicochemical and pharmacokinetic properties of pharmacologically potent compounds. Prodrugs are changed and form active drugs inside the body through enzymatic or non-enzymatic reactions. This article describes the potent secondary metabolites from natural sources that are amenable to prodrug design. A fair number of them have issues of bioavailability and metabolism, resulting in less efficacy, and a prodrug strategy is required to improve their efficacy. This has come into the spotlight recently by achieving encouraging results in most cases. Many phytochemicals are not preferred due to their low solubility, decreased bioavailability, and adverse effects, but these problems can be overcome with the formation of prodrugs of such compounds. Several techniques can be used to synthesize these prodrugs, including biotransformation, electrophilic substitution, esterification, biomodulation, covalent conjugation, and complex formation. A detailed study of the problem encountered by the bioactive of natural origin, the process of formation of their prodrugs and the advancements produced due to the generation of such prodrugs are discussed in this review & takes the focus on the foremost utilization of the prodrug approach, comprising the capability to enhance oral absorption & aqueous solubility, enhance lipophilicity, increase active transport, and also attain site-selective application.
Keywords: Bioavailability, Biomodulation, Esterification, Permeability, Prodrugs, and Toxicity
INTRODUCTION: A prodrug is a chemically modified form of a pharmacologically active molecule that remains inactive or less active until it transforms within the body. This conversion may occur through enzymatic or non-enzymatic pathways, releasing the active drug at the site of action 1.
A prodrug typically consists of two components: the parent drug and a moiety. While the promoiety itself does not exhibit therapeutic activity, it is strategically selected to improve critical drug properties such as solubility, absorption, stability, or targeting ability 1–4.
The major purpose of prodrug design is to overcome shortcomings of bioactive compounds, such as poor solubility in water or lipids, limited tissue selectivity, unfavorable taste, local irritation, rapid metabolism, or systemic toxicity. By modifying such compounds into prodrugs, their pharmacokinetic and pharmacodynamic profiles absorption, distribution, metabolism, excretion (ADME) can be enhanced, leading to safer and more effective therapies. Plant-derived secondary metabolites are of particular interest because they provide a wide range of bioactive molecules with diverse chemical scaffolds.
However, many of these phytochemicals suffer from inadequate solubility, poor permeability, instability, and rapid metabolism, limiting their therapeutic potential. Prodrug strategies, including polymer conjugation and nanodrug formation, have been investigated to improve these limitations, especially in the field of cancer therapy 5.
This review highlights the design, synthesis, and applications of prodrugs derived from natural compounds, emphasizing examples with anticancer, antimicrobial, and antioxidant potential.
Categories of Prodrugs: Prodrugs can be classified according to the mechanism by which they are activated inside the body 6–12.
Bioprecursor Prodrugs: These do not carry an additional promoiety. Instead, they rely on metabolic transformation by enzymes to generate the active compound.
Carrier-Linked Prodrugs: Here, the drug is attached to a carrier group (commonly esters, phosphates, or amides) that enhances solubility or permeability. The carrier is cleaved enzymatically or chemically after administration to release the active drug.
Schiff Base Prodrugs: These involve imine or enamine linkages with primary amines. They increase lipophilicity and reduce ionization, improving membrane transport. They are generally stable but can hydrolyze in-vivo to release the parent amine.
N-Mannich Base Prodrugs: Created by derivatizing amides or amines with a Mannich base, these derivatives improve aqueous solubility and lipophilicity. They are stable under certain conditions but release the parent drug in-vivo through hydrolysis.
Formation of Trisindolines from Isatin: Isatin, also called tribulin, is an indole-based molecule known for antitumor, antiviral, anti-HIV, and antitubercular activities 13. However, due to its limited potency, researchers have designed trisindolines, which are condensation products of isatin with indoles. These compounds exhibit a broad range of bioactivities, including anticancer, antimicrobial, antifungal, and antioxidant effects.
The synthesis of trisindolines usually involves acid-catalyzed electrophilic substitution reactions between indole and isatin. The reaction mechanism proceeds via an intermediate (3-hydroxy-3-indolyl-2-indolone) which, upon further reaction with indole, yields 3,3-di(3-indolyl)-2-indolone 14, 15.
Different catalysts are used for these reactions:
- Mineral acids such as sulphuric 16, phosphoric 17, and tungstic acids 18.
- Organic acids, including p-toluenesulfonic acid, acetic acid, and sulfamic acid 19, 20.
- Halogen-based catalysts, e.g., iodine 21 or N-bromosuccinimide 22.
- Metal-based catalysts, such as aluminium sulfate or ceric ammonium nitrate, are cost-effective but require careful handling 23.
Problems Faced During the Administration of Drugs, Along with Their Solutions: Many drugs fail during formulation because of poor solubility, inadequate stability, or undesirable sensory properties. Prodrug design offers an efficient solution to such limitations 24.
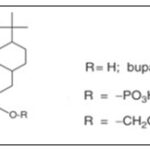
Poor Solubility: Buparvaquone suffers from poor water solubility. By converting it into phosphate ester derivatives, solubility and skin permeability were improved, enhancing its oral and topical bioavailability Fig. 1 25–28.
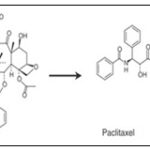
Paclitaxel is another poorly soluble drug. The prodrug isotaxel, created via O,N-acyl migration, displays improved solubility and bioavailability Fig. 2 24, 25, 29.
Dapsone derivatives linked with amino acids showed improved aqueous solubility while retaining antimicrobial activity 19.
FIG. 1: BUPARVAQUONE AND ITS PRODRUGS
FIG. 2: PACLITAXEL AND ITS PRODRUGS ISOTAXEL
Unpleasant Taste: Erythromycin-A has a strong bitter taste, leading to poor compliance in children. Its tasteless prodrug, erythromycin ethylsuccinate, undergoes hydrolysis after administration, releasing the active drug while masking its taste 24, 30.
Toxicity: 5-Fluorouracil (5-FU), a widely used anticancer drug, causes severe myelotoxicity when administered directly. Safer prodrugs such as capecitabine, UFT, and S-1 have been developed, which provide similar or better efficacy with reduced toxicity 31. Polymeric derivatives of 5-FU further enhance controlled release and biocompatibility 32.
Weak Pharmacological Activity: Lawsone and its derivatives demonstrate limited potency, but their metal complexes and prodrug forms enhance biological activity 33. Plumbagin, another naphthoquinone, has been converted into prodrugs through chemical modification or metal chelation, improving anticancer activity while reducing toxicity 34.
Strategies for Prodrug Formation: Prodrugs can be engineered using a variety of chemical and biotechnological approaches:
Polymeric Prodrugs: Active molecules covalently linked to polymers, offering better stability, site-specific delivery 35, and controlled release 36.
RSSH-Releasing Prodrugs: Designed to release persulfides, which play roles in redox balance and cytoprotection 37.
Phenazine Derivatives (Iodinin, Myxin): Modified into carbonate or carbamate prodrugs to improve solubility and anticancer selectivity 38.
Chiral Compound Formation: Use of enzymatic or organometallic catalysis to generate enantioselective prodrugs of compounds such as lawsone 39.
Amine Addition to Sesquiterpene Lactones: Masks reactive groups and improves solubility 40.
Amino Acid Conjugates: Enhance bioavailability and blood-brain barrier penetration 41.
Single-Chain Lipid Conjugates:
Example: acyclovir lipid derivatives that self-assemble into nanoparticles with extended half-life.
Prodrugs Derived from Various Phytochemicals: Prodrug discovery from natural sources is categorized by the identification, elucidation, derivatization, and chemical modification of secondary metabolites obtained from natural resources, which undergo biotransformation before reaching the binding site and cellular receptors to produce the desired therapeutic effect, overcoming the limitation of the physiological barrier. The role of phytochemicals as potential prodrugs or therapeutic substances against various diseases has come into the spotlight in very recent years. Thankfully, the huge mass of inspiring and capable results of the in-vitro activity of many phenolic compounds from plant extracts against several diseases was identified 41. The various natural products used as prodrugs are:
Prodrug of Allicin: Allicin is a sulfur-containing natural compound having several biological properties that are accountable for the characteristic smell and taste of freshly cut or crushed garlic (Allium sativum) 42, 43. Allicin has anti-microbial, anti-cancerous, and immunomodulatory effects and is used in the treatment of hypercholesterolemia. It is very effective against various health problems, but it has stability issues in the human body.
Allin Fig. 3B is a prodrug of allicin Fig. 3A, an unstable compound that is transformed into diallyl disulfide. This sulfoxide prodrug is a natural component of fresh garlic that is much more stable than allicin and is transformedinto its bioactive form by the enzyme allinase. This prodrug, after conversion, can act as an anti-bacterial and anti-hyperlipidemic agent 42.
FIG. 3: (A) ALLICIN (B) PRODRUG, ALLIN
Prodrug of Curcumin: Curcumin (diferuloyl-methane) is an orange-yellow polyphenolic compound isolated from the rhizomes of turmeric with a varied range of pharmacological effects, like anti-cancerous, antiviral, anti-arthritic, antioxidant, and immunomodulatory effects, but it holds a lot of bioavailability issues 44, 45.
The synthesis of curcumin diethyl disuccinate is attained via one-step esterification between curcumin and succinic acid monoethyl ester chloride using 4-(N,N-dimethylamino) pyridine as a catalyst 46. Curcumin diethyl disuccinate, a prodrug of curcumin, has enhanced chemical and metabolic stability; hence, the oral bioavailability is increased, which can lead to the enhanced anti-proliferative activity of the drug. This prodrug is used widely in colon and breast cancer 47–49. Curcumin diglutaric acid, an ester prodrug of curcumin, has the potential to be established as an anti-inflammatory agent due to its enhanced solubility and stability 50.
Prodrug of Epigallocatechin-3-gallate: Epigallocatechin-3-gallate is the chief catechin found in green tea. It has antioxidant effects, cancer chemoprevention, enhances cardiovascular health, improves weight loss, defends the skin from the damage caused by ionizing radiation, and others 51. The drug has a wide range of effects but has low efficacy. So, the epigallocatechin-3-gallate Fig. 4A is converted to a prodrug, proepigallocatechin-3-gallate Fig. 4B by acetylation can alleviate mouse laser-induced CNV leakage and reduced CNV area by down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1-type macrophage/microglia polarization as well as endothelial cell viability, proliferation, migration and tube formation, denoting a novel potential therapy for the age-related macular degeneration 52.
FIG. 4: (A) EPIGALLOCATECHIN-3-GALLATE (B) PRODRUG, PROEPIGALLOCATECHIN-3-GALLATE
Prodrug of Podophyllotoxin: Podophyllin, an ethanolic extract of Podophyllum peltatum or P. emodi, is a great source of the aryltetralin-type lignan, podophyllotoxin. Podophyllotoxin Fig. 5A is a potent anti-cancerous drug, but due to the adverse effects, it is now preferably used in the form of a prodrug. The adverse effects were due toits low water solubility and high toxicity. A prodrug of podophyllotoxin, 7-hydroxymethyl-2,3-dihydro-1H-cyclopent-a[b] chromene-1-one Fig. 5B, is prepared by esterification of the original drug through biotransformation and nucleophilic addition of a sulfhydryl group to the position of the α,β-unsaturated ketone, which produces phenol anions that promote rapid 1,6-elimination of the intermediate, resulting in the formation of the prodrug. This prodrug is more water-soluble, less toxic, and is considered a promising candidate in cancer therapy 53, 54.
FIG. 5: (A) PODOPHYLLOTOXIN (B) PRODRUG, 7-HYDROXYMETHYL-2,3-DIHYDRO-1H-CYCLOPENT-A[B]CHROMENE-1-ONE
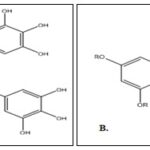
Prodrug of Quercetin and Resveratrol: Quercetin is the chief flavonoid present in various vegetables and fruits. Quercetin possesses a diversity of biological activities, including antioxidant, prevention of oxidation of low-density lipoproteins in-vitro, and anticarcinogenic activities. The major drawback of the drug is its bioavailability issues 55–58. The esterification of quercetin results in the formation of a prodrug, 3-O-acylquercetins, and quercetin-3-O-palmitate. These prodrugs have effective antioxidant activity and are associated with liposomal membranes. Resveratrol (3, 4′, 5-trihydroxystilbene) is a naturally occurring phytoalexin formed by some spermatophytes, such as grapevines, in response to injury. Resveratrol may provide cardiovascular protection and also possesses anti-inflammatory and anticancer properties 59. But the compound faces bioavailability issues. The alkylation and structural modification of resveratrol Fig. 6A as the 3,5-diglucosyl-resveratrol and piceid Fig. 6B and 3-butylated resveratrol inhibit the proliferation and cause death by both apoptosis and necrosis of a human colon carcinoma cell line 60. The prodrug resveratrol has increased bioavailability due to the improved chemical stability and solubility in water, and hence it is more effective than the original drug 5, 61.
FIG. 6: (A) RESVERATROL (B) PRODRUG, PICEID
Prodrug of Tropoflavin: 7, 8-Dihydroxyflavone (7,8-DHF), also known as tropoflavin, a small flavonoid, is a natural polyphenolic compound found in several vegetables, fruits, and tree leaves. 7, 8-DHF potentially inhibited cancer growth, proliferation, invasion, and metastasis, as well as various brain-related diseases 62. Tropoflavin Fig. 7A is characterized by poor oral bioavailability and a shorter half-life. A prodrug of tropoflavin named R-13 Fig. 7B was synthesized by ester or carbamate group alteration on the catechol ring in the parent moiety, which has significantly increased oral bioavailability and half-life, which is proven to be more beneficial than the parent drug. This prodrug is used against various brain-related disorders 5, 63.
FIG. 7: (A) TROPOFLAVIN (B) PRODRUG, R-13
Prodrug of Baicalein: Baicalein flavonoid, is a hydrophilic drug used as a neuroprotective, anti-depressant, anti-cancerous, antioxidant, anti-inflammatory, and hepatoprotective agent that is incapable of crossing the BBB 64. A prodrug is formed from the baicalein by various methods, including endogenous transporters (e.g., carrier-mediated prodrug transport), macromolecular delivery mechanisms (e.g., receptor-mediated prodrug transport), and gene-directed enzyme prodrug therapy. This prodrug is more lipophilic due to which allows it can cross the BBB easily & is more effective. This prodrug is used as a neuroprotective and anti-depressant agent 5, 65, 66.
Prodrug of Cinnamaldehyde: Trans-Cinnamaldehyde is an aldehydic terpenoid obtained from cinnamon (Cinnamomum zyelanicum). It is used as an anti-bacterial, carminative, cardiotonic, etc. 67. Cinnamaldehyde Fig. 8A is a chemically unstable and water-soluble drug that hinders its potential bioactivity. A prodrug formed by the interaction of tryptamine with cinnamaldehyde is known as TRY-CA (Tryptamine-Cinnamaldehyde) Fig. 8B, which is much more chemically stable and much more biologically active than cinnamaldehyde itself. This prodrug is used in the treatment of glioma 5, 68.
FIG. 8: (A) CINNAMALDEHYDE (B) PRODRUG, TRY-CA
Prodrug of Colchicine: Colchicine is an alkaloid obtained from the extract of Colchicum (Colchicum autumnale). Colchicine is being used as an anti-fibrotic, anti-inflammatory, anti-gout agent, etc., but has limited use due to the hepatotoxicity that it produces 69. Colchicine was hydroxyl-functionalized by substituting the N-acetyl moiety with an N-2-hydroxyacetyl moiety. Afterward, the hydroxyl group was reacted with methoxy PEG-acetic acid to get the hydrolyzable polymeric colchicinoid prodrug. This prodrug has reduced systemic toxicity, therefore opening the door for its application in cancer therapy and as an anti-viral agent 70, 71.
Prodrug of α-methylene-γ-lactone: α-methylene-γ-lactone is a sesquiterpene lactone mostly found in the Compositae family, which has anti-microbial, anti-proliferative, anti-leukemic, and anti-cancerous properties 72. It has deprived aqueous solubility and a non-selective binding property as a Michael acceptor at undesired targets. A prodrug of α-methylene-γ-lactone is developed by adding an amine to the parent drug to mask this group from nucleophiles and enhance solubility, hence getting a more potent drug 73.
TABLE 1: SOME NATURAL BIOACTIVE COMPOUNDS AND THEIR PRODRUGS
| S. no. | Bioactive compound | Prodrug | Problem overcome | Pharmacological use |
| 1 | Allicin | Allin | Increased stability | Anti-bacterial and Anti-hyperlipidemic activity |
| 2 | Curcumin | Curcumin diethyl disuccinate | Increased stability and bioavailability | Colon and breast cancer |
| 3 | Curcumin | Curcumin diglutaric acid | Improved solubility and stability | Anti-inflammatory agent |
| 4 | Epigallocatechin-3-gallate | proepigallocatechin-3-gallate | Increased activity | Age related macular degeneration |
| 5 | Podophyllotoxin | 7-hydroxymethyl-2,3-dihydro-1H-cyclopent-a[b]chromene-1-one | More water soluble and less toxic | Anti-cancerous agent |
| 6 | Quercetin | 3-O-acylquercetins and quercetin-3- O-palmitate | Increased bioavailability | Antioxidant effect |
| 7 | Resveratrol | 3,5-diglucosyl-resveratrol, piceid octanoate and 3-butylated resveratrol | Increased bioavailability, improved chemical stability and solubility in water | Colon cancer |
| 8 | 7,8-dihydroxyflavone | R-13 | Increased oral bioavailability and half life | Various brain related disorders |
| 9 | Baicalein | - | More lipophilic and crosses blood brain barrier | Neuroprotective and anti-depressant |
| 10 | Cinnamaldehyde | TRY-CA | Chemically stable | Treatment of glioma |
| 11 | Colchicine | - | Reduced systemic toxicity | Anti-inflammatory, anti-viral and anti-cancerous |
| 12 | α-methylene-γ-lactone | - | Increased solubility | Anti-cancerous agent |
CONCLUSION: The prodrug approach offers a versatile and powerful strategy to optimize natural bioactive compounds with limited pharmaceutical applicability. By modifying phytochemicals, researchers can overcome issues such as poor solubility, rapid metabolism, instability, and toxicity. Examples across multiple natural products demonstrate improved bioavailability, better targeting, and reduced adverse effects.
Future directions in prodrug research include the design of intelligent linkers and spacers, polymeric and nanocarrier-based systems, and the integration of computational modeling to predict activation and optimize pharmacokinetics. Given their potential, natural product-derived prodrugs will likely remain central in drug discovery and development.
Statement & Declaration: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. No funding was applicable for this review article. The authors show no conflict of interest. All the authors have equally contributed to the work.
ACKNOWLEDGEMENTS: Nil
CONFLICT OF INTEREST: The authors here by confirm no conflict of interest.
REFERENCES:
- Aeila ASS, Rahaman ST and Praveen V: A Brief Overview on Prodrug and Its Design. IJPPR Human 2019; 17(1): 225–31.
- Rautio J, Laine K, Gynther M and Savolainen J: Prodrug approaches for CNS delivery. AAPS Journal 2008; 10(1): 92–102.
- Brunton LL, Lazo JS, Parker KL and McGraw‐Hill: The pharmacological basis of therapeutics. Occup Environ Med 2007; 64(8): 2.
- Han HK and Amidon GL: Targeted prodrug design to optimize drug delivery. AAPS J 2000; 2(1): 48–58.
- Rassu G, Sorrenti M, Catenacci L, Pavan B, Ferraro L and Gavini E: Conjugation, prodrug, and co-administration strategies in support of nanotechnologies to improve the therapeutic efficacy of phytochemicals in the central nervous system. Pharmaceutics 2023; 15(6): 1578.
- Bundgaard H and Johansen M: Hydrolysis of N-Mannich bases and its consequences for the biological testing of such agents. Int J Pharm 1981; 9: 7–16.
- Simplicio AL, John M, Clancy LM and Gilmer JF: Beta- aminoketones as prodrugs with pH-controlled activation. Int J Pharm 2007; 336: 208–14.
- Silva A, Chung MC, Castro LF, Guido RVC and Ferreira LI: Advances in prodrug design. Mini Reviews on Medicinal Chemistry 2005; 5: 893–914.
- Lavis LD: Ester bonds in prodrugs. ACS Chem Biol 2008; 3: 1–3.
- Wu KM and Farrelly J: Regulatory perspectives of type II prodrug development and time-dependent toxicity management: Nonclinical pharm/tox analysis and the role of comparative toxicology. Toxicology 2007; 236: 1–6.
- Testa B and Mayer JM: Hydrolysis in drug and prodrug metabolism: chemistry, biochemistry and enzymology. Weinheim, Federal Republic of Germeny: Wiley-VCH 2003.
- Jana S, Mandlekar S and Marathe P: Prodrug design to improve pharmacokinetic and drug delivery properties. Challenges to the Discovery Scientists [Internet]. Vol. 17, Current Medicinal Chemistry 2010. Available from: http://en.wikipedia.org/
- Wati FA, Santoso M, Moussa Z, Fatmawati S, Judeh A and Fadlana A: Chemistry of trisindolines: natural occurrence, synthesis and bioactivity. Royal Society of Chemistry 2021; 11: 25381–421.
- Rad-Moghadam K and Gholizadeh S: Selective and efficient synthesis of 3-indolyl-2-oxindoles under catalysis of LiClO4. Iran Journal of Catalyst 2014; 4: 41–7.
- Rad-Moghadam K, Sharifi-Kiasaraie M and Taheri-Amlashi H: Synthesis of symmetrical and unsymmetrical 3,3-di(indolyl)indolin-2-ones under controlled catalysis of ionic liquids. Tetrahedron 2010; 66: 2316–21.
- Sayed MT, Mahmoud K, Hilgroth A and Fakhr MI: Synthesis of novel indolo-spirocyclic compounds. Journal of Hetrocyclic Chemistry 2015; 53: 188–96.
- Esmaielpour M, Akhlaghinia B and Jahanshashi R: Green and efficient synthesis of aryl/alkylbis (indolyl) methanes using Expanded Perlite-PPA as a heterogeneous solid acid catalyst in aqueous media. Journal of Chemical Sciences 2017; 129: 313–28.
- Patel GM and Deota PT: Tungstic acid catalysed synthesis of 3,3-bis(1H-indole-3-yl) indolin-2-one derivatives. Heterocycl Comm 2013; 19: 421–4.
- Shirini F and Khaligh NG: Succinamide-N-sulfonic acid catalysed synthesis of bis(indolyl) methane and coumarin derivatives under mild conditions. Chinese Journal of Catalysts 2013; 34: 1890–6.
- Brahmachari G and Banerjee B: Facile and one-pot access of 3,3-bis(indol-3-yl)indolin-2-ones and 2,2-bis (indol-3-yl) acenaphthylen-1(2H)-one derivatives via an eco-friendly pseudo-multi component reaction at room temperature using sulfamic acid as an organo-catalyst. ACS Sustainable Chemical Engineering 2014; 2: 2802–12.
- Paira P, Hazara A, Kumar S, Paira R and Sahu KB: Efficient synthesis of 3,3-dihetroaromatic oxindole analouges and their in-vitro evaluation for spermicidal potential. Bioorganic & Medicinal Chemistry Lett 2009; 19: 4786–9.
- Haung W, Nang L, Li X, Ma Y and Liang D: Bromine/para-toluenesulfonic acid-catalysed synthesis of 3,3-bis(indole-3-yl) indoline-2-(1H)-ones by condensing indoles with isatins. Chin J Chem 2015; 33: 1167–72.
- Kamal A, Srikanth YV V, Khan MNA, Shaik TB and Ashraf M: Synthesis of 3,3-diindolyl oxyindoles efficiently catalyzed by FeCl3 and their in-vitro evaluation for anticancer activity. Biorganic Medical Chemistry Lett 2010; 20: 5229–31.
- Testa B: Prodrug objectives and design. Chemistry, Molecular Sciences and Chemical Engineering 2007; 5(2): 1009–41.
- Testa B: Comprehensive Medicinal Chemistry II 2007; 1009–1041.
- Pochopin NL, Charman WN and Stella VJ: Amino acid derivatives of dapsone as water-soluble prodrugs. Int J Pharm 1995; 121(2): 157–67.
- Eilenberg H, Pnini-Cohen S, Rahamim Y, Sionov E, Segal E and Carmeli S: Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. J Exp Bot 2010; 61(3): 91–922.
- Mantyla A, Garnier T, Rautio J, Nevalainen T, Vepsalainen J and Koskinen A: Synthesis in-vitro evaluation and Anti-leishmanial activity of water soluble prodrugs of buparvaquone. J Med Chem 2004; 47: 188–95.
- Skwarczynski M, Noguchi M, Hirota S, Sohma Y, Kimura T and Hayashi Y: Development of first photoresponsive prodrug of paclitaxel. Bioorg Med Chem 2006; 16(17): 4492–6.
- Bhadra P, GA M and JB: Design, synthesis, and evaluation of stable and taste-free erythromycin proprodrugs. J Med Chem 2005; 48: 3878–84.
- Dufrasne F, Gelbcke M, Nève J, Kiss R and Kraus JL: quinone methides and their prodrugs: a subtle equilibrium between cancer promotion, prevention, and cure. Current Medical Chemistry 2011; 18: 3995–4011.
- Li M, Liang Z, Sun X, Gong T and Zhang Z: A polymeric prodrug of 5-fluorouracil-1-acetic acid using a multi-hydroxyl polyethylene glycol derivative as the drug carrier. PLoS One 2014; 9(11): 1–13.
- Salunke-Gawali S, Pereira E, Dar UA and Bhand S: Metal complexes of hydroxynaphthoquinones: Lawsone, Bis-Lawsone, Lapachol, Plumbagin and Juglone. Journal of Molecular Structure: THEOCHEM 2017; 17: 3–59.
- Tan M, Liu Y, Luo X, Chen Z and Liang H: Antioxidant Activities of Plumbagin and Its Cu (II) Complex. Hindawi 2011; 5.
- Chu H, Sun R, Li JS, Li X, Wang W and Teng L: Polymeric prodrug by supramolecular polymerization. React Funct Polym 2023; 191: 105654–9.
- Chis AA, Arseniu AM, Morgovan C, Dobrea CM, Frum A and Juncan AM: Biopolymeric Prodrug Systems as Potential Antineoplastic Therapy. Pharmaceutics 2022; 14(9): 1773–81.
- Xu BX, Hu TY, Du JB, Xie T, Xu YW and Jin X: In pursuit of feedback activation: New insights into redox-responsive hydropersulfide prodrug combating oxidative stress. Redox Biol 2024; 72: 103130–5.
- Viktorsson EO, Aesoy R, Støa S, Lekve V, Døskeland SO and Herfindal L: New prodrugs and analogs of the phenazine 5,10- dioxide natural products iodinin and myxin promote selective cytotoxicity towards human acute myeloid leukemia cells. RSC Med Chem 2021; 1–12.
- Pasha MA, Anebouselvy K and Ramachary DB: Lawsone as synthon in the catalytic asymmetric reactions. Tetrahedron 2022; 117–118: 132793–9.
- Woods JR, Mo H, Bieberich AA, Alavanja T and Colby DA: Amino-derivatives of the sesquiterpene lactone class of natural products as prodrugs. Vol. 4, Med Chem Comm Royal Society of Chemistry 2013; 27–33.
- Thukral S, Chawla P, Sharma A and Chawla V: Recent Advancement in Prodrugs 2020; 31–42.
- Borlinghaus J, Albrecht F, Gruhlke M, Nwachukwu I and Slusarenko A: Allicin: Chemistry and Biological Properties. Molecules 2014; 19(8): 12591–618.
- Slusarenko AJ, Patel A and Portz D: Control of plant diseases by natural products: Allicin from garlic as a case study. Eur J Plant Pathol 2008; 121: 313–22.
- Ratnatilaka Na Bhuket P, El-Magboub A, Haworth IS and Rojsitthisak P: Enhancement of curcumin bioavailability via the prodrug approach: challenges and prospects. Eur J Drug Metab Pharmacokinet 2017; 42(3): 341–53.
- Hongyu Z, Christopher SB and Shile H: The Targets of Curcumin. Curr Drug Targets 2011; 12(3): 332–47.
- Muangnoi C, Bhuket PRN, Jithavech P, Wichitnithad W, Srikun O and Nerungsi C: Scale-up synthesis and in vivo anti-tumor activity of curcumin diethyl disuccinate, an ester prodrug of curcumin, in HepG2-xenograft mice. Pharmaceutics 2019; 11(8): 1–14.
- Wichitnithad W, Nimmannit U, Wacharasindhu S and Rojsitthisak P: Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules 2011; 16: 1888–900.
- Bhunchu S, Rojsitthisak P, Muangnoi C and Rojsitthisak P: Curcumin diethyl disuccinate encapsulated in chitosan/alginate nanoparticles for improvement of its in-vitro cytotoxicity against MDA-MB-231 human breast cancer cells. Pharmazie 2016; 71: 691–700.
- Muangnoi C, Ratnatilaka Na Bhuket P, Jithavech P, Supasena W, Paraoan L and Patumraj S: Curcumin diethyl disuccinate, a prodrug of curcumin, enhances anti-proliferative effect of curcumin against HepG2 cells via apoptosis induction. Sci Rep 2019; 9(1): 1–9.
- Phumsuay R, Muangnoi C, Wasana PWD, Hasriadi, Vajragupta O and Rojsitthisak P: Molecular insight into the anti-inflammatory effects of the curcumin ester prodrug curcumin diglutaric acid in-vitro and in-vivo. Int J Mol Sci 2020; 21(16): 1–16.
- Nagle DG, Ferreira D and Zhou YD: Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006; 67(17): 1849–55.
- Xu J, Tu Y, Wang Y, Xu X, Sun X and Xie L: Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomedicine and Pharmacotherapy 2020; 121: 109606.
- Kusari S, Zühlke S and Spiteller M: Chemometric evaluation of the anti-cancer pro-drug podophyllotoxin and potential therapeutic analogues in Juniperus and Podophyllum species. Phytochemical Analysis 2011; 22(2): 128–43.
- Li S, Li X, Lu Y, Hou M, Xu Z and Li B: A thiol-responsive and self-immolative podophyllotoxin prodrug for cancer therapy. Tetrahedron Lett 2021; 71: 153044.
- Toshihiro Okamoto: Safety of quercetin for clinical application (Review). Int J Mol Med 2005; 16: 275–8.
- GCJustino, Santos M, Canario S, Borges C, Florencio M and Mira L: Plasma quercetin metabolites: structure-antioxidant activity relationships. ABB 2004; 432: 109–21.
- Whalley C De, Rankin S, Holt J, Jessup W and Leake D: Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biochem Pharmacol 1990; 39: 1743–50.
- Pereira M, Grubbs C, Barnes L, Li H, Olson G and Eto I: Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a] anthracene-induced mammary cancer in rats. Carcinogenesis 1996; 17: 1305–11.
- Fremont L: Biological effects of resveratrol. Life Sci 2000; 66(8): 663–73.
- Biasutto L and Zoratti M: Prodrugs of quercetin and resveratrol: a strategy under development. Curr Drug Metab 2014; 15: 77–95.
- Intagliata S, Modica M, Santagati L and Montenegro L: Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants 2019; 8: 244.
- Ju WS, Seo SY, Yu JO, Kim JS, Lee SR and Choo YK: 7,8-Dihydroxyflavone down-regulates the expression level of ganglioside GD3 and induces the dysfunction of mitochondrial membrane permeabilization in melanoma cells. Korean Society for Glycoscience 2022; 7: 89.
- Chen C, Wang Z, Zhang Z, Liu X, Kang S and Zhang Y: The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc Natl Acad Sci USA 2018; 115: 578–83.
- Gupta S, Buttar HS, Kaur G and Tuli HS: Baicalein: promising therapeutic applications with special reference to published patents. Pharm Pat Anal 2022; 11(1): 2021–7.
- Tarrago T, Kichik N, Claasen B, Prades R, Teixido M and Giralt E: Baicalin, a Prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg Med Chem 2008; 16: 7516–24.
- Jia X, Jia M, Yang Y, Wang D, Zhou F and Zhang W: Synthesis of novel baicalein amino acid derivatives and biological evaluation as neuroprotective agents. Molecules 2019; 24: 3647.
- Doyle AA and Stephens JC: A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019; 139: 104405.
- Zhao H, Xie Y, Yang Q, Cao Y, Tu H and Cao W: Pharmacokinetic study of cinnamaldehyde in rats by GC-MS after oral and intravenous administration. J Pharm Biomed Anal 2014; 89: 150–7.
- Niel E and Scherrmann JM: Colchicine today. Joint Bone Spine 2006; 73(6): 672–8.
- Crielaard BJ, Wal S van der, Lammers T, Le HT, Hennink WE and Schiffelers RM: A polymeric colchicinoid prodrug with reduced toxicity and improved efficacy for vascular disruption in cancer therapy. Int J Nanomedicine 2011; 6: 2697–703.
- Richter M, Boldescu V, Graf D, Streicher F, Dimoglo A and Bartenschlager R: Synthesis, Biological Evaluation and Molecular Docking of Combretastatin and Colchicine Derivatives and their hCE1-Activated Prodrugs as Antiviral agent. Chemistry Europe 2019; 14(4): 469–83.
- Gach K and Janecka A: α-Methylene-γ-lactones as a Novel Class of Anti-leukemic Agents. Anti-cancerous agent in Medicinal Chemistry 2014; 14(5): 688–94.
- Woods JR, Mo H, Bieberich AA, Alavanja T and Colby DA: Amino-derivatives of the sesquiterpene lactone class of natural products as prodrugs. Medicinal Chemistry Community 2013; 4: 27–33.
How to cite this article:
Chandwani A and Shah MB: A comprehensive review on prodrugs of bioactive of natural origin. Int J Pharmacognosy 2025; 12(10): 780-88. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.12(10).780-88.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
780-788
617 KB
321
English
IJP
Ankita Chandwani and Mamta B. Shah *
Department of Pharmacognosy, L. M. College of Pharmacy, Navrangpura, Ahmedabad, Gujarat, India.
mbshah2007@rediffmail.com
22 September 2025
28 October 2025
30 October 2025
10.13040/IJPSR.0975-8232.IJP.12(10).780-88
31 October 2025