PHYTOCHEMICAL SCREENING AND HEPATOPROTECTIVE EFFICACY OF LEAVES EXTRACTS OF ANNONA SQUAMOSA AGAINST PARACETAMOL INDUCED LIVER TOXICITY IN RATS
HTML Full TextPHYTOCHEMICAL SCREENING AND HEPATOPROTECTIVE EFFICACY OF LEAVES EXTRACTS OF ANNONA SQUAMOSA AGAINST PARACETAMOL INDUCED LIVER TOXICITY IN RATS
S. Rajeshkumar *, B. Tamilarasan and V. Sivakumar
PG and Research Department of Biochemistry, Adhiparasakthi College of Arts and Science, Kalavai, Vellore - 632506, Tamil Nadu, India.
ABSTRACT: Phytochemicals are the best remedies to all kind of diseases and disorders throughout the world. There are a lot of plants, and its sources are playing a role as a heart, liver and kidney savers. In our present investigation, the famous fruit tree Annona squamosa (Annonaceae) leaves extract used for the hepatoprotective activity. The phytochemicals present in the aqueous leaves extracts were analyzed using preliminary biochemical analysis, and functional groups are characterized using Fourier Transform Infrared Spectrophotometer (FT-IR). The animals were divided into six groups, in that apart from the first group (normal control), five groups are induced with paracetamol (acetaminophen) with standard drug and two plant drug extracted by aqueous one is induced control. The increasing of enzymes and bilirubin, triglycerides and cholesterol and decreasing protein shows the liver damage. These levels are changed into normal range indicates efficiency of our plant drug. The histopathalogical results are also confirmed our hepatoprotective capability is good.
| Keywords: |
A. squamosa, Hepatoprotective, FT-IR, Phytochemicals, Herbal medicine
INTRODUCTION: There is a lot of plants have been used for the hepatoprotective activity by carbon tetrachloride, paracetamol or acetaminophen, ethanol, thioacetamide, rifampicin, isoniazid and pyrazinamide induced liver toxicity. The plats have hepatoprotective capability against paracetamol-induced liver toxicity are flowers of Calotropis procera 1, fruit extract of 2, Rhazya stricta, Balanitis aegyptiaca and Haplophylum tuberculatum 3, Swertia chirata 4, vegetable and its leaves extracts of Brassica oleracea 5, Aerva lanata 6, Tabebuia rosea and Solanum pubescent 7,
Ichnocarpus frutescens 8, chenopodium album linn 9, ethanolic extract of Aerva sanguinolenta 10, ethanolic extract of Chamomile capitula 11, Amorphophallus paeoniifolius tubers 12, polyherbal formulations of a hydroalcoholic extract of Tinospora cordifolia and ethanol extract of Boerhavia diffuse, Phyllanthus amaraus, Euphorbia hirta, Wedelia chinesis 13.
Taxonomic Classification of Annona squamosa:
Division: Magnoliophyta
Class: Magnoliopsida
Sub class: Magnoliidae
Order: Magnoliales
Family: Annonaceae
Genus: Annona L.
Species: squamosa
For the past few decades, plants like Annona squamosa are used for the treatment of various applications like anti-diabetic, anti-inflammatory, hepatoprotective, antibacterial, anti-fungal and antitumor activity. With these points under consideration, the present study was carried out to investigate the hepatoprotective effect of aqueous leaves extracts of Annona squamosa on paracetamol-induced hepatotoxicity in rats. In this present investigation To isolate the aqueous extracts of leaf of Annona squamosa and to study their phytochemical constitution by FT-IR studies, and assess the hepatoprotective activity of the aqueous leaves extracts of Annona squamosa and by studying the parameters such as changes in the activity of AST, ALT, and the level of bilirubin, Alterations in the level of triglycerides, total protein and total cholesterol and histological changes in normal and experimental albino rats.
FIG. 1: ANNONA SQUAMOSA LEAVES
TABLE 1: BIOMEDICAL APPLICATIONS OF A. SQUAMOSA
| S. no. | Medicinal uses | Plant Parts and phytochemicals | Reference |
| 1 | Analgesic and anti-inflamatory | bark | 14 |
| 2 | Antibacterial activity against
B. subtilis B. cereus, B. megaterium, Staphylococcus aureus S. b-hemolytic, Sarcina lutea, E. coli, S. dysenteriae, S. shiga, S. flexneriae, S. sonnei, Salmonella typhi, P. aeruginosa, Klebsiella sp. |
Annotemoyin-1
Annotemoyin-2 Squamocin Cholesteryl glucopyranoside |
15 |
| 3 | Antioxidant | Leaves (aqueous) | 16 |
| 4 | Antiulcer | leaves | 17 |
| 5 | Hypoglycaemic and anti-diabetic | leaves | 18 |
| 6 | Vasorelaxant activity
(The treatment of cerebral vasospasm and hypertension for good peripheral circulation) |
Seeds (Octapeptide, cyclosquamosin B) | 19 |
| 7 | Molluscicidal activity
(Schistosoma mansoni) |
Seed, root, stem, bark, and leaves | 20 |
| 8 | Geno toxic efficacy | Seed (isosquamosin) | 21 |
| 9 | Antitumour
[Multiple drug resistant (MDR) tumor cell lines]
|
asimicin18, squamocin18, squamocin-D18, desacetyluvaricin18, Iso desacetyluvaricin18, squamostatin-D18, squamostatin-E18, squamostatin- B18, squamostatin-A18, 12,15-cis-squamostatin-A19, 4- deoxyannoreticuin20, and cis-4-deoxyannoreticuin20 | 22 |
| 10 | Insecticidal activity
(Housefly Musca domestica) |
leaves, bark
and seeds |
23 |
MATERIALS AND METHODS:
Preparation of Extracts: The collected leaves of A. squamosa were washed thoroughly with running water to remove adherent impurities, chopped and air dried under shade for 7 days and then crushed into coarse powder. The dried leaves were pulverized in an electric grinder. The dried leaves were extracted in Soxhlet extractor with water separately for 8 h. The extracts were then reduced to a dark-colored molten mass by using rotary evaporator under reduced pressure. The extracts were used for phytochemical analysis and stored at 4 °C for further use.
Experimental Design: The rats were randomly divided into five groups of 6 animals each.
Group I (Control): Control rats received orally distilled water.
Group II (Induced): Induced rats, orally received paracetamol (2 g/kg body weight) dissolved in water for 7 days.
Group III (Paracetamol + Silymarin): Standard rats, orally received paracetamol (2 g/kg body weight), followed by silymarin (100 mg/kg body weight) dissolved in water for 7 days.
Group IV (Paracetamol + AAS): Treated rats, orally received paracetamol (2 g/kg body weight), followed by an aqueous extract of A. squamosa leaf (500mg/kg body weight) dissolved in water for 7 days.
Group V (Paracetamol + AAS): Treated rats, orally received paracetamol (2 g/kg body weight), followed by an aqueous extract of A. squamosa leaf (1000mg/kg body weight) dissolved in water for 7 days.
Ethical clearance was obtained from the Institutional Animal Ethical Committee, CPCSEA, India (Reg No.282/ac/09/CPCSEA).
Biochemical Analysis: Animals of all the groups were sacrificed by cervical decapitation on the 8th day. Blood samples of each group were collected separately into sterilized dry centrifuge tubes and allowed to coagulate for 30 min at 37 ºC. The clear serum obtained after centrifugation was used for the estimation of serum alanine aminotransferase, serum aspartate aminotransferase, serum bilirubin, serum protein, cholesterol, and triglycerides.
Histopathology: A portion of liver tissue in each group was fixed in 10% formalin (formalin diluted to 10% with normal saline) and processed for histopathology. After paraffin embedding, and block marking, a serial section of 5μm thicknesses were made, stained with hematoxylin and eosin and examined under the microscope.
RESULTS AND DISCUSSION:
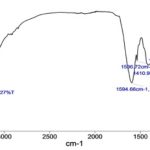
Phytochemical Constituents of A. squamosa: The presence of phytochemicals such as alkaloids, glycoside, flavonoids, saponins, and phenolic compounds was confirmed by qualitative phytochemical analysis shown in Table 2. These are the key constituents used as liver savers. Fig. 2 and Table 3 show the FT-IR spectrum of the A. squamosa leaves aqueous extract. In that, the functions groups such as phenol and methanol, benzene, ethylamine, nitromethane, methyl formate, and diethyl ether, diethyl ether and methyl formate were confirmed by its absorption ranges and types of vibration.
TABLE 2: PHYTOCHEMICAL CONSTITUENTS OF A. SQUAMOSA
| Constituents | Aqueous Extract |
| Flavonoids | + |
| Triterpene | - |
| Glycoside | + |
| Saponins | + |
| Phenolic compounds | + |
| Steroids | - |
| Alkaloids | + |
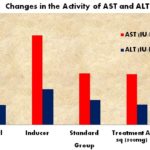
Hepatoprotective Activity of Annona squamosa: Plants are major pharmaceutical resources having a lot of applications like antidiabetic 24, kidney savers and nephroprotective agents 25, 26, 27 hepato-protective materials 5, 13 heart medicines like cardioprotective agents 28, 29 antimicrobials such as antibacterials and antifungal agents against disease-causing infectious pathogens 30. And finally, the plants are playing important role in nanoparticles synthesis nowadays 31, 32, 33, 34. The effects of aqueous extract of A. squamosa on serum enzymes were studied, and the results were given in Fig. 3 and Table 3. It is well known that acetaminophen is used as a hepatotoxic agent on various animals.
Administration of acetaminophen to rats produced hepatotoxicity showed by a significant increase in the serum enzymes levels of ALT, AST and serum triglycerides and cholesterol in comparison to control group. The aqueous extract of A. squamosa showed a significant decrease in the serum enzymes ALT, AST and ALP but also significantly improved the triglycerides and cholesterol level when compared to the acetaminophen compared to control groups. There were raise in GSH content of liver after treatment with A. squamosa aqueous extract Standard drug, silymarin (25 mg/kg) also exhibited similar results significantly.
FIG. 2: FT-IR SPECTRUM OF A. SQUAMOSA LEAVES AQUEOUS EXTRACT
TABLE 3A: FT-IR OF A. SQUAMOSA
| Absorption ranges | Types of vibration | Functional group |
| 3339.51 | Hydrogen-bonded O-H stretch | Phenol and methanol |
| 1594.66 | C-C=C symmetric Stretch | Benzene |
| 1566.72 | N-H bend | Ethylamine |
| 1410.96 | N=O bend | Nitromethane |
| 1236.01 | C-O stretch | Methyl formate and diethyl ether |
| 1042.74 | C-O stretch | Diethyl ether and methyl formate |
TABLE 3B: CHANGES IN THE ACTIVITY OF AST AND ALT IN EXPERIMENTAL ANIMALS
| Animal Groups | AST (IU/L) | ALT (IU/L) |
| Group I | 100.67 ± 4.33 | 44.83 ± 1.80 |
| Group II | 200.50 ± 10.17 | 80.67 ± 4.82 |
| Group III | 115.67 ± 4.51 | 56.33 ± 1.76 |
| Group IV | 113.00 ± 4.91 | 45.33 ± 2.21 |
| Group V | 108.33 ± 4.23 | 49.83 ± 1.94 |
| Level of Significance (p) | < 0.05 | < 0.05 |
FIG. 3: CHANGES IN THE ACTIVITY OF AST AND ALT IN EXPERIMENTAL ANIMALS
Table 4 and Fig. 4 present the changes in the level of serum total bilirubin, protein, Cholesterol and Triglycerides. Here, paracetamol-induced liver damage was characterized by increased level of bilirubin, Cholesterol, and Triglycerides and they return to normal level after 7 days of treatment with aqueous extracts from A. squamosa leaf. Hyperlipidemia is the most common disorder in liver diseases. Damaging of liver cells by paracetamol may cause the hyperlipidemia clearly shown in total cholesterol and triglycerides level. These higher levels reduced near the normal and standard level which indicates our plant capability against liver damage. The liver is known to play an important role in the serum protein synthesis, is the source of plasma albumin and fibrinogen and also a and b-globulin.
The liver is also concerned with the synthesis of g-globulin. The serum albumin level is low in liver damage conditions. The paracetamol was damaging the hepatic cells its due to hypoalbuminaemia. 28. The aqueous extracts from A. squamosa leaves may increase the protein levels in the serum. It indicates that the protection of hepatic cells from paracetamol induction.
TABLE 4: CHANGES IN THE LEVELS OF SERUM BILIRUBIN, TOTAL PROTEIN, CHOLESTEROL AND TRIGLYCERIDES IN CONTROL AND EXPERIMENTAL RATS
| Parameters | Group
I |
Group
II |
Group
III |
Group
IV |
Group
V |
Level of
Significance (p) |
| Bilirubin (mg/dL) | 0.15 ± 0.06 | 0.20 ± 0.15 | 0.15 ± 0.06 | 0.16 ± 0.02 | 0.17 ± 0.03 | < 0.05 |
| Total Protein (g/dL) | 7.49 ± 0.29 | 7.09 ± 0.23 | 7.42 ± 0.28 | 7.30 ± 0.33 | 7.41 ± 0.38 | < 0.05 |
| Cholesterol (mg/dL) | 121.67±5.62 | 349.50±15.86 | 115.00±4.23 | 160.00±5.24 | 145.87±5.52 | < 0.05 |
| Triglycerides (mg/dL) | 108.5± 5.14 | 219.83±10.20 | 112.33±5.40 | 145.00 ± 6.02 | 134.17±5.62 | < 0.05 |
Fig. 4: Changes in the activity of Bilirubin, total protein, TGL and cholesterol
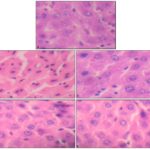
Histopathology: The hepatoprotective effect of the A. squamosa aqueous extract against paracetamol-induced injury was confirmed by histopathological examination. The hepatocyte damage caused by paracetamol (acetaminophen) is shown in histopathological studies of liver Fig. 5B. Administration of A. squamosa aqueous leaves extract along with acetaminophen excreted significant protection against toxicity and showed normal appearance in the liver. The effect of standard drug silymarin and plant extracts on hepatocytes clearly showed the protective effects of aqueous extracts of A. squamosa against paracetamol-induced liver damage Fig. 5C-5E.
FIG. 5: HISTOPATHOLOGICAL EXAMINATION FOR THE HEPATOPROTECTIVE ACTIVITY OF A. SQUAMOSA IN PARACETAMOL TREATED WISTAR RATS. (A) NORMAL CONTROL (B) PARACETAMOL TREATED RAT (C) STANDARD RATS, ORALLY RECEIVED PARACETAMOL FOLLOWED BY SILYMARIN (D) TREATED RATS, ORALLY RECEIVED PARACETAMOL FOLLOWED BY AQUEOUS EXTRACT OF A. SQUAMOSA LEAF (500 mg/kg BODY WEIGHT) (E) TREATED RATS, ORALLY RECEIVED PARACETAMOL FOLLOWED BY AQUEOUS EXTRACT OF A. SQUAMOSA LEAF (1000 mg/kg BODY WEIGHT)
CONCLUSION: A. squamosa fruits are very popular for their taste. In traditionally A. squamosa leaves are used for various biomedical activities. The hepatoprotective effects of A. squamosa leave aqueous extract investigated under acetaminophen-induced hepatotoxicity in male albino rats. The treatment of A. squamosa suppressed the increase of SGOT (Serum glutamate oxaloacetate trans-aminase) and SGPT (Serum glutamate pyruvate transaminase) in the serum of acetaminophen injured rats. When compared with the normal and inducer the significant changes were identified in the activity of the total protein, total bilirubin, total cholesterol, and triglycerides indicates the A. squamosa have a lot of phytochemicals which protect the hepatic injury. In some cases, the A. squamosa leaves extracts are have a good result when compared to standard drug silymarin. Finally, we conclude that our plant is the best remedy for our liver. It has a lot of pharmacological properties for its phytochemicals.
ACKNOWLEDGEMENT: The Authors would like to thank Dr. A. Mohamed sadiq, Principal, APCAS, Kalavai for his support and valuable suggestions.
CONFLICT OF INTEREST: We declare that we have no conflict of interest.
REFERENCES:
- Setty SR, Qureshi AA, Viswanath Swamy AHM, Patil T, Prakash T, Prabhu K and Veeran Gouda A: Hepato-protective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia 2007; 78: 451-454.
- Gilani AH and Khalid HJ: Preventive and curative effects of Berberis aristata fruit extract on paracetamol- and CCl4-induced hepatotoxicity. Phytotherapy Research 1995; 9: 489-494.
- Ali AH, Bashir AK and Rasheed RA: Effect of the traditional medicinal plants Rhazya stricta, Balanitis aegyptiaca & Haplophylum tuberculatum on paracetamol-induced hepatotoxicity in mice. Phytotherapy Research 2001; 15: 598-603.
- Karan M, Vasisht K and Handa SS: Antihepatotoxic activity of chirata on paracetamol and galactosamine- induced hepatotoxicity in rats. Phytotherapy Research 1999; 13: 95-101.
- Bhavani R, Kotteeswaran R and Rajeshkumar S: Hepato-protective effect of Brassica oleracea vegetable and its leaves in paracetamol-induced liver damage in albino rats. International Journal of Chem Tech Research 2014; 6(7): 3705-3712.
- Manokaran S, Jaswanth A, Sengottuvelu S, Nandhakumar J, Duraisamy R, Karthikeyan D and Mallegaswari R: Hepatoprotective activity of Aerva lanata against paracetamol induced hepatotoxicity in rats. Research J Pharm and Tech 2008; 1: 398-400.
- Hemamalini K, Ramya Krishna V, Bhargav A and Vasireddy U: Hepatoprotective activity of Tabebuia rosea and Solanum pubescens against paracetamol-induced hepatotoxicity in rats.
- Dash DK, Yeligar VC, Nayak SS, Ghosh T, Rajalingam D, Sengupta P, Maiti BC and Maity TK: Evaluation of hepatoprotective and antioxidant activity of Ichnocarpus frutescens (Linn.) R.Br. on paracetamol-induced hepato-toxicity in rats. Tropical Journal of Pharmaceutical Research 2007; 6(3): 755-765.
- Pal A, Banerjee B, Banerjee T, Masih M and Pal K: Hepato-protective activity of Chenopodium albumplant against paracetamol-induced hepatic injury in rats. International Journal of Pharmacy and Pharmaceutical Sciences 2011; 3(3).
- Lalee A, Bhattacharya B, Das M, Mitra D, Kaity S, Bera S and Samanta A: Hepatoprotective activity of ethanolic extract of Aerva sanguinolenta (Amaranthaceae) against paracetamol-induced liver toxicity on Wistar rats. NSHM Journal of Pharmacy and Healthcare Management 2012; 03: 57-65
- Gupta AK and Misra N: Hepatoprotective activity of aqueous ethanolic extract of Chamomile capitula in paracetamol intoxicated albino rats. American Journal of Pharmacology and Toxicology 2006; 1(1): 17-20.
- Hurkadale PJ, Shelar PA, Palled SG, Mandavkar YD and Khedkar AS: Hepatoprotective activity of Amorphophallus paeoniifolius tubers against paracetamol-induced liver damage in rats. Asian Pacific Journal of Tropical Biomedicine 2012; S238-S242.
- Sivakumar V, Rajan MSD, Sadiq AM and Rajeshkumar S: Hepatoprotective effect of polyherbal formulations in paracetamol induced hepatic damaged experimental rats. International Research Journal of Pharmaceutical and Biosciences 2014; 1(1): 30-35.
- Chavan MJ, Wakte PS and Shinde DB: Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa bark. Phytomedicine 2010; 17: 149-151.
- Rahman MM, Shahnaj P, Haque ME, Islam ME, Mohammad A and Mosaddik B: Antimicrobial and cytotoxic constituents from the seeds of Annona squamosa. Fitoterapia 2005; 76: 484-489.
- Gupta RK, Kesari AN, Diwakarc S, Tyagia A, Tandona V, Chandra R and Watal G: In-vivo evaluation of anti-oxidant and anti-lipidomics potential of Annona squamosa aqueous extract in Type 2 diabetic models. J of Ethnopharmacology 2008; 118: 21-25.
- Yadav DK, Singh N, Dev K, Sharma R, Sahai M, Palit G and Maurya R: Anti-ulcer constituents of Annona squamosa Fitoterapia 2011; 82: 666-675.
- Gupta RK, Kesari AN, Murthy PS, Chandra R, Tandon V and Watal G: Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa in experimental animals. Journal of Ethnopharmacology 2005; 99: 75-81.
- Arayaa H, Sahaib M, Singhb S, Singhb AK, Yoshidac M, Harad N and Fujimotod Y: Squamocin-O1 and squamocin-O2 new adjacent bis-tetrahydrofuran acetogenins from the seeds of squamosa. Phytochemistry 2002; 61: 999-04.
- Dos Santos AF and Sant'Ana AEG: Molluscicidal properties of some species of Annona. Phytomedicine 2001; 8(2): 115-120.
- Grover P, Singh SP, Prabhakar PV, Reddy UA, Balasubramanyam A, Mahboob M, Rahman MF and Misra S: In-vivo assessment of genotoxic effects of Annona squamosa seed extract in rats. Food and Chemical Toxicology 2009; 47: 1964-1971.
- Yang H, Zhang N, Li X, Chen J and Cai B: Structure-activity relationships of diverse annonaceous acetogenins against human tumor cells. Bioorganic & Medicinal Chemistry Letters 2009; 19: 2199-2202.
- Leatemia JA and Isman MB: Insecticidal Activity of crude seed extracts of Annona spp., Lansium domesticum and Sandoricum koetjape against Lepidopteran larvae. Phytoparasitica 2004; 32(1): 30-37.
- Bhavani R and Rajeshkumar S: Anti-hyperglycemic activity of alcoholic leaf extract of Aegle marmelos (Linn.) on alloxan-induced diabetic rats. International Journal of Pharma Sciences and Research 2013; 5(3): 56-62
- Ramesh K, Sudakar M, Manohar S and Rajeshkumar S: Nephroprotective activity of ethanolic extract of Orthosiphon stamineus leaves on ethylene glycol induced urolithiasis in Albino rats. International Journal of Pharm Tech Research. 2014; 6(1):403-408.
- Nandhini BS, Rojalakshmi B, Shobana R and Rajeshkumar S: Effect of noni (Morinda citrifolia) extract on the treatment of ethylene glycol and ammonium chloride induced kidney disease. International Journal of Pharma Sciences and Research 2014; 5(6): 249-256.
- Ramesh K, Paari E and Rajeshkumar S: In-vivo nephroprotective activity of Kalanchoe pinnata leaves on ethylene glycol induced urolithiasis in Albino rats. Indo American Journal of Pharmaceutical Research 2014; 4(7): 3093-3098.
- Sivakumar V and Rajeshkumar S: Screening of cardio-protective effect of Terminalia arjuna bark in isoproterenol - Induced myocardial infarction in experimental animals International Journal of Pharma Sciences and Research 2014; 5(6): 262-268.
- Bhavani R, Shobana R and Rajeshkumar S: Cardio-protective activity of Pithecellobium dulce flower and fruit pulp aqueous extracts International Journal of Pharmaceutical Sciences and Research 2014; 6(3): 82-89.
- Rajeshkumar S: Antimicrobial effect of King of bitter Andrographis paniculata and traditional herb Aegle marmelos against clinical pathogens. International Journal of Pharm Tech Research 2014-2015; 7(2): 325-329.
- Vanaja M, Paulkumar K, Rajeshkumar S, Jobitha G, Malarkodi C, Sivakavinesan M and Annadurai G: Herbal plant synthesis of antibacterial silver nanoparticles by Solanum trilobatum and its characterization 2014; International Journal of Metals http://dx.doi.org/10.1155/ 2014/692461.
- Paulkumar K, Gnanajobitha G, Vanaja M, Rajeshkumar S, Malarkodi C and Annadurai G: Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against plant pathogens. The Scientific World Journal 2013. http://dx.doi. org/10.1155/ 2014/829894
- Vanaja M, Rajeshkumar S, Paulkumar K, Gnanajobitha G, Malarkodi C and Annadurai G: Phytoreduction of silver ions using stem and root extract of Coleus aromaticus International Journal of Materials and Biomaterials Applications 2013; 3(1): 1-4.
- Gnanajobitha G, Paulkumar K, Vanaja M, Rajeshkumar S, Malarkodi C, Annadurai G and Kannan C: Fruit mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. Journal of Nanostructures in Chemistry 2013; 3(67): 1-6.
How to cite this article:
Rajeshkumar S, Tamilarasan B and Sivakumar V: Phytochemical screening and hepatoprotective efficacy of leaves extracts of Annona squamosa against paracetamol induced liver toxicity in rats. Int J Pharmacognosy 2015; 2(4): 178-85. doi link: http://dx.doi.org/10.13040/ IJPSR.0975-8232.IJP.2(4).178-85.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
6
178-85
787
3105
English
IJP
S. Rajeshkumar*, B. Tamilarasan and V. Sivakumar
PG and Research Department of Biochemistry, Adhiparasakthi College of Arts and Science, Kalavai, Vellore, Tamil Nadu, India.
ssrajeshkumar@hotmail.com
14 January 2015
15 March 2015
29 March 2015
10.13040/IJPSR.0975-8232.IJP.2(4).178-85
01 April 2015